The cytoplasmic zinc finger protein ZPR1 accumulates in the nucleolus of proliferating cells - PubMed (original) (raw)
The cytoplasmic zinc finger protein ZPR1 accumulates in the nucleolus of proliferating cells
Z Galcheva-Gargova et al. Mol Biol Cell. 1998 Oct.
Free PMC article
Abstract
The zinc finger protein ZPR1 translocates from the cytoplasm to the nucleus after treatment of cells with mitogens. The function of nuclear ZPR1 has not been defined. Here we demonstrate that ZPR1 accumulates in the nucleolus of proliferating cells. The role of ZPR1 was examined using a gene disruption strategy. Cells lacking ZPR1 are not viable. Biochemical analysis demonstrated that the loss of ZPR1 caused disruption of nucleolar function, including preribosomal RNA expression. These data establish ZPR1 as an essential protein that is required for normal nucleolar function in proliferating cells.
Figures
Figure 1
The cytoplasmic ZPR1 protein redistributes to the nucleus of mitogen-activated cells. A431 cells were incubated without (STARVED) and with 100 nM EGF for 15 min. The cells were processed for immunofluorescence microscopy using an antibody to ZPR1.
Figure 2
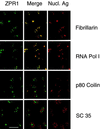
Nuclear ZPR1 accumulates in the nucleolus. Activated HEp-2 cells were examined by laser scanning confocal microscopy using antibodies to ZPR1 (FITC, green) and nuclear antigens (rhodamine, red). In the merged image, fibrillarin and RNA Pol I show extensive colocalization (yellow), whereas p80 coilin and SC35 splicing factor show extensive segregation (green and red). Bar, 35 μm. Fibrillarin and RNA Pol I are markers for the nucleolus.
Figure 3
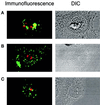
ZPR1 is not an integral component of the fibrillar or granular components of the nucleolus. Proliferating HEp-2 cells were treated (4 h) with 0.1 μg/ml actinomycin D or with 25 μg/ml of the adenosine analogue DRB, fixed, and processed for immunofluorescence microscopy using antibodies to ZPR1 (FITC, green) and antibodies that stain the fibrillar component (fibrillarin; rhodamine, red) and the granular component (B23; rhodamine, red) of the nucleolus. Cells treated with actinomycin D were stained with ZPR1 and fibrillarin (A). Cells treated with DRB were stained with antibodies to ZPR1 and fibrillarin (B) or ZPR1 and B23 (C). A photomicrograph of the differential interference contrast (DIC) image is presented in each panel.
Figure 4
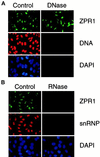
RNA is required for the nucleolar localization of ZPR1. HEp-2 cells were grown on microscopic slides, permeabilized with 0.1% Triton X-100 for 3 min on ice, washed, and digested (60 min at 37°C) with 0.1 mg/ml DNase I in PBS containing 5 mM MgCl2 or with 0.1 mg/ml RNase A in PBS. The cells were washed in PBS and processed for indirect immunofluorescence. Buffers without enzymes served as negative controls. A human antibody to snRNP or a monoclonal antibody to DNA was used to monitor the efficiency of DNase I and RNase A digestions, respectively. The effect of DNase I (A) and RNase A (B) is presented.
Figure 5
The ZPR1 gene is highly conserved in mammals and yeast. The sequences of the human, mouse, and yeast (S. pombe and S. cerevisiae) ZPR1 proteins were compared using the PILE-UP program (version 7.2; Wisconsin Genetics Computer Group, Madison, WI). Residues that are identical with human ZPR1 are indicated with a period. Gaps were introduced to optimize the alignment (–). The two zinc fingers are overlined, and the Cys residues are indicated with asterisks. The sequence of mouse ZPR1 (GenBank accession number U41287) has been reported (Galcheva-Gargova et al., 1996). The human and yeast ZPR1 protein sequences were deduced from the nucleotide sequence of HeLa cDNA clones isolated from a λZAP II phage library (Stratagene) and from the sequence of yeast genomic clones. The sequences of the human ZPR1 cDNA, the S. pombe zpr1+ gene, and the S. cerevisiae ZPR1 gene have been deposited in GenBank with accession numbers AF019767, AF019768, and AF019769, respectively.
Figure 6
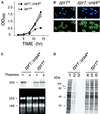
The zpr1+, gene is essential for cell viability. (A) The S. pombe zpr1+ gene was disrupted by homologous recombination. The structure of the zpr1+ genomic locus and the disrupted gene (zpr1::ura4+) is presented schematically. (B) Southern blot analysis of the diploid yeast transformants. The genomic DNA was restricted with _Bgl_II and probed with a random-primed fragment of the zpr1+ genomic locus (2.9-kb _Xba_I fragment). The wild-type zpr1+ allele was identified in wild-type yeast (strain 480; lane 1). The disrupted zpr1::ura4+ allele (3 kb) was identified in some (lanes 2 and 4) but not all (lane 3) transformants. (C) The heterozygous diploid yeast strain TE630 (zpr1+/zpr1::ura4+) was sporulated, and the tetrads were dissected. The viability of the spores was examined by growth on agar plates supplemented with uracil. (D) The heterozygous (zpr1+/zpr1::ura4+) diploid yeast strain TE630 was transformed with the plasmid pREP41 or the plasmid pREP41-zpr1, selected on minimal agar plates without leucine and uracil, and sporulated, and haploid yeast were selected on minimal media supplemented with adenine. No viable haploid yeast (zpr1::ura4+) were obtained from diploid yeast transformed with pREP41. However, the zpr1 expression vector pREP41-zpr1 complemented the lethal phenotype of the disrupted zpr1+ gene. Complementation was observed in experiments using S. pombe zpr1+, S. cerevisiae ZPR1, and murine ZPR1. Repression of the nmt promoter in the pREP plasmid with thiamine decreased the growth of the complemented ZPR::ura4+ haploid strains, but not the wild-type zpr1+ haploid strain transformed with pREP41-zpr1.
Figure 7
Loss of zpr1+ function causes depletion of the rRNA precursor and decreased protein translation. (A) Wild-type haploid S. pombe (zpr1+) and the zpr1::ura4+ disrupted strain were transformed with the plasmid pREP41-zpr1 and grown in minimal liquid medium. The cultures were divided into two flasks in the absence (open symbols) and presence (closed symbols) of thiamine, respectively. Thiamine is a repressor of the nmt promoter located in the pREP plasmid. The growth of the cultures was monitored by measurement of the optical density at 595 nm. (B) The morphology of the yeast grown in the presence of thiamine (12 h) was examined by phase-contrast microscopy. DNA stained with 4,6-diamidino-2-phenylindole was visualized by epifluorescence. Bar, 10 μm. (C) Northern blot analysis of RNA isolated from the zpr1+ and the zpr1::ura4+ disrupted S. pombe strains transformed with the plasmid pREP41-zpr1. The yeast were grown in the absence and presence of the repressor thiamine (12 h). Ten micrograms of RNA isolated from these yeast were examined by denaturing agarose gel electrophoresis. The 25 and 18S mature rRNA were detected by staining with ethidium bromide (bottom panel). The 35S rRNA precursor was detected by Northern analysis using a 5′-ETS probe. An autoradiogram of the dried blot is shown (top panel). (D) Wild-type and zpr1::ura4+ disrupted strains were grown in the absence (lanes 1 and 4) and presence (lanes 2, 3, 5, and 6) of the repressor thiamine (12 h). The cells were diluted to the same density (0.2 OD595), labeled with [35S]methionine (150 μCi/ml) for 3 h, and harvested. The labeling with [35S]methionine was performed in the absence (lanes 1, 2, 4, and 5) and presence (lanes 3 and 6) of thiamine. Extracts prepared from the yeast were examined by SDS-PAGE and autoradiography.
Similar articles
- Interaction of ZPR1 with translation elongation factor-1alpha in proliferating cells.
Gangwani L, Mikrut M, Galcheva-Gargova Z, Davis RJ. Gangwani L, et al. J Cell Biol. 1998 Dec 14;143(6):1471-84. doi: 10.1083/jcb.143.6.1471. J Cell Biol. 1998. PMID: 9852145 Free PMC article. - Binding of zinc finger protein ZPR1 to the epidermal growth factor receptor.
Galcheva-Gargova Z, Konstantinov KN, Wu IH, Klier FG, Barrett T, Davis RJ. Galcheva-Gargova Z, et al. Science. 1996 Jun 21;272(5269):1797-802. doi: 10.1126/science.272.5269.1797. Science. 1996. PMID: 8650580 - NoBP, a nuclear fibroblast growth factor 3 binding protein, is cell cycle regulated and promotes cell growth.
Reimers K, Antoine M, Zapatka M, Blecken V, Dickson C, Kiefer P. Reimers K, et al. Mol Cell Biol. 2001 Aug;21(15):4996-5007. doi: 10.1128/MCB.21.15.4996-5007.2001. Mol Cell Biol. 2001. PMID: 11438656 Free PMC article. - Structural insights into the interaction of the evolutionarily conserved ZPR1 domain tandem with eukaryotic EF1A, receptors, and SMN complexes.
Mishra AK, Gangwani L, Davis RJ, Lambright DG. Mishra AK, et al. Proc Natl Acad Sci U S A. 2007 Aug 28;104(35):13930-5. doi: 10.1073/pnas.0704915104. Epub 2007 Aug 17. Proc Natl Acad Sci U S A. 2007. PMID: 17704259 Free PMC article. - High-fat diet-associated cognitive decline: Is zinc finger protein 1 (ZPR1) the molecular connection?
Chittilla M, Akimbekov NS, Razzaque MS. Chittilla M, et al. Curr Res Physiol. 2021 Oct 2;4:223-228. doi: 10.1016/j.crphys.2021.09.004. eCollection 2021. Curr Res Physiol. 2021. PMID: 34746842 Free PMC article. Review.
Cited by
- Investigation of variants identified in caucasian genome-wide association studies for plasma high-density lipoprotein cholesterol and triglycerides levels in Mexican dyslipidemic study samples.
Weissglas-Volkov D, Aguilar-Salinas CA, Sinsheimer JS, Riba L, Huertas-Vazquez A, Ordoñez-Sánchez ML, Rodriguez-Guillen R, Cantor RM, Tusie-Luna T, Pajukanta P. Weissglas-Volkov D, et al. Circ Cardiovasc Genet. 2010 Feb;3(1):31-8. doi: 10.1161/CIRCGENETICS.109.908004. Epub 2009 Dec 11. Circ Cardiovasc Genet. 2010. PMID: 20160193 Free PMC article. - The role of nuclear bodies in gene expression and disease.
Morimoto M, Boerkoel CF. Morimoto M, et al. Biology (Basel). 2013 Jul 9;2(3):976-1033. doi: 10.3390/biology2030976. Biology (Basel). 2013. PMID: 24040563 Free PMC article. - Association of a genetic variant of the ZPR1 zinc finger gene with type 2 diabetes mellitus.
Tokoro F, Matsuoka R, Abe S, Arai M, Noda T, Watanabe S, Horibe H, Fujimaki T, Oguri M, Kato K, Minatoguchi S, Yamada Y. Tokoro F, et al. Biomed Rep. 2015 Jan;3(1):88-92. doi: 10.3892/br.2014.379. Epub 2014 Nov 7. Biomed Rep. 2015. PMID: 25469254 Free PMC article. - Association between the MLX interacting protein-like, BUD13 homolog and zinc finger protein 259 gene polymorphisms and serum lipid levels.
Aung LH, Yin RX, Wu JZ, Wu DF, Wang W, Li H. Aung LH, et al. Sci Rep. 2014 Jul 3;4:5565. doi: 10.1038/srep05565. Sci Rep. 2014. PMID: 24989072 Free PMC article. - Cyclophilin A peptidyl-prolyl isomerase activity promotes ZPR1 nuclear export.
Ansari H, Greco G, Luban J. Ansari H, et al. Mol Cell Biol. 2002 Oct;22(20):6993-7003. doi: 10.1128/MCB.22.20.6993-7003.2002. Mol Cell Biol. 2002. PMID: 12242280 Free PMC article.
References
- Abelson HT, Penman S. Selective interruption of RNA metabolism by chemotherapeutic agents. Handbook Exp Pharmacol. 1975;38:571–581.
- Fu X-D, Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990;343:437–441. - PubMed
- Galcheva-Gargova Z, Konstantinov KN, Wu I-H, Klier FG, Barrett T, Davis RJ. Binding of zinc finger protein ZPR1 to the epidermal growth factor receptor. Science. 1996;272:1797–1802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases