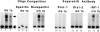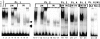Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication - PubMed (original) (raw)
Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication
Y Honda et al. J Exp Med. 1998.
Abstract
We have previously observed that HIV-1 replication is suppressed in uninflamed lung and increased during tuberculosis. In vitro THP-1 cell-derived macrophages inhibited HIV-1 replication after infection with Mycobacterium tuberculosis. Suppression of HIV-1 replication was associated with inhibition of the HIV-1 long terminal repeat (LTR) and induction of ISGF-3, a type I interferon (IFN)-specific transcription factor. Repression of the HIV-1 LTR required intact CCAAT/enhancer binding protein (C/EBP) sites. THP-1 cell-derived macrophages infected with M. tuberculosis, lipopolysaccharide, or IFN-beta induced the 16-kD inhibitory C/EBPbeta isoform and coincidentally repressed HIV-1 LTR transcription. C/EBPbeta was the predominant C/EBP family member produced in THP-1 macrophages during HIV-1 LTR repression. In vivo, alveolar macrophages from uninflamed lung strongly expressed inhibitory 16-kD C/EBPbeta, but pulmonary tuberculosis abolished inhibitory C/EBPbeta expression and induced a novel C/EBP DNA binding protein. Therefore, in vitro, proinflammatory stimulation produces an IFN response inhibiting viral replication by induction of a C/EBPbeta transcriptional repressor. THP-1 cell-derived macrophages stimulated with type I IFN are similar to alveolar macrophages in the uninflamed lung in vivo. In contrast, the cellular immune response in active pulmonary tuberculosis disrupts this innate immunity, switching C/EBP expression and allowing high level viral replication.
Figures
Figure 3
Nucleotide sequence of the HIV-1 LTR. C/EBP binding sites determined by DNA footprinting are shown in boxes (from reference 19). The mutations introduced to abolish C/EBP binding are shown below the wild-type sequence. The NF-κB sites are underlined. The transcription start site is marked (arrow).
Figure 1
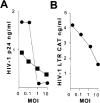
HIV-1 replication and LTR function after infection with M. tuberculosis. (A) HIV-1 replication is suppressed by M. tuberculosis infection. Monocytic THP-1 cells differentiated to macrophages by PMA were infected with HIV-1 BaL (•) or HIV-1 NL4-3 (▪). HIV-1 replication was measured by p24 production at day 7 after infection. (B) HIV-1 LTR is repressed by M. tuberculosis infection. BF-24 cells containing a stable integrated HIV-1 LTR CAT construct were differentiated with PMA and infected with M. tuberculosis at various moi. CAT activity was measured 48 h later.
Figure 2
ISGF-3 binding to ISRE DNA in THP-1 macrophages. Treatment with IFN-α and infection with M. tuberculosis induces ISGF-3 expression. Lane 1, Unstimulated macrophages do not express ISGF-3. Lane 2, Stimulation with IFN-α (IFN) induces an ISRE binding complex. Lane 3, Infection with M. tuberculosis (Tb) produces a comigrating complex (arrow). Lanes 4–6, The ISRE–protein complex was disrupted by excess unlabeled ISRE-containing competitor. Lanes 7–9, The ISRE– protein complex was not disrupted by excess unlabeled nonspecific oligonucleotide competitor. Lanes 10–15, The ISRE–protein complex was disrupted by antibodies to Stat-1 and Stat-2. Lanes 16–18, The ISRE– protein complex was not disrupted by antibodies to IRF-1. A nonspecific DNA–protein complex is shown at the bottom of each panel for reference (double arrow).
Figure 4
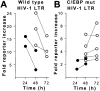
Time course of HIV-1 LTR promoter activity in stably transfected THP-1 cells. (A) Wild-type HIV-1 LTR. M. tuberculosis (•) or LPS (○) produces a striking decline in reporter activity in THP-1 macrophages between 24 and 72 h. (B) HIV-1 LTR with mutated (mut) C/EBPβ sites. M. tuberculosis (•) or LPS (○) produces a moderate increase in reporter activity in THP-1 macrophages.
Figure 5
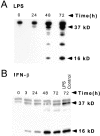
C/EBPβ expression and HIV-1 LTR reporter activity of THP-1 macrophages after LPS or IFN-β stimulation. (A) LPS induces inhibitory 16-kD C/EBPβ in the nuclear extracts between 48 and 72 h. (B) Inhibitory 16-kD C/EBPβ is increased 3 h after IFN treatment of differentiated THP-1 cells and is maximally induced after 48 h. The level of inhibitory C/EBPβ produced by LPS stimulation is the same as that produced by IFN-β. (C) The HIV-1 LTR is repressed after 1 U/ml IFN-β in differentiated BF-24 cells. There is a 50% decline in LTR activity after 3 h and a 95% decline in LTR activity at 72 h.
Figure 5
C/EBPβ expression and HIV-1 LTR reporter activity of THP-1 macrophages after LPS or IFN-β stimulation. (A) LPS induces inhibitory 16-kD C/EBPβ in the nuclear extracts between 48 and 72 h. (B) Inhibitory 16-kD C/EBPβ is increased 3 h after IFN treatment of differentiated THP-1 cells and is maximally induced after 48 h. The level of inhibitory C/EBPβ produced by LPS stimulation is the same as that produced by IFN-β. (C) The HIV-1 LTR is repressed after 1 U/ml IFN-β in differentiated BF-24 cells. There is a 50% decline in LTR activity after 3 h and a 95% decline in LTR activity at 72 h.
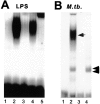
Figure 6
C/EBPβ binding to the HIV-1 LTR NRE in THP-1 macrophages. (A) Cells predominantly express C/EBPβ after LPS stimulation. Lane 1, Unstimulated THP-1 cells with wild-type NRE probe. Lane 2, Wild-type NRE binding is increased 10-fold after stimulation with PMA and LPS. Lane 3, Competition with 100-fold excess unlabeled wild-type NRE probe disrupts the NRE–protein complex. Lane 4, 100-fold excess unlabeled NRE mutated in the C/EBP site does not disrupt the NRE–protein complex. Lane 5, Antibody to C/EBPβ supershifts >95% of the NRE–protein complex. (B) THP-1 macrophages infected with M. tuberculosis (M. tb.) express C/EBP-specific and C/EBP-nonspecific NRE–protein complexes. Lane 1, Wild-type NRE probe only. Lane 2, Wild-type NRE binding in THP-1 macrophage extracts after M. tuberculosis infection produces a band similar to LPS stimulation (arrow) and another more rapidly migrating NRE–protein complex (double arrow). Lane 3, C/EBP mutant NRE probe only. Lane 4, C/EBP-mutated NRE probe produces a single complex with the same mobility as the rapidly migrating wild-type NRE–protein complex (double arrow) with extracts of _M. tuberculosis_–infected THP-1 macrophages.
Figure 7
Transcription factor expression in BAL cells from a normal control and six patients with pulmonary tuberculosis. (A) Inhibitory 16-kD C/EBPβ is strongly expressed in BAL cells from uninflamed lung and is downregulated in pulmonary tuberculosis. Lane 1, The inhibitory 16-kD C/EBPβ is strongly expressed in a normal control (NL). Lane 2, Adherent BAL cells (Ad) from an uninvolved lobe of an AIDS patient with tuberculosis strongly express inhibitory 16-kD C/EBPβ. Adherent BAL cells are >95% alveolar macrophages. Lane 3, Nonadherent cells (Non) which are 80–90% lymphocytes have little inhibitory 16-kD C/EBPβ expression. Lanes 2, 4, 6, 8, 10, and 12 show that the inhibitory 16-kD C/EBPβ is strongly expressed in uninvolved lobes (UN) of six patients with tuberculosis. Four are HIV-1 infected and two are HIV-1 negative. Lanes 5, 7, 9, 11, and 13 show that the inhibitory 16-kD C/EBPβ is markedly downregulated in the involved lobes (IN) of these tuberculosis patients. Lane 14, There is an increase in 16-kD C/EBPβ in the involved lobe after 2 wk of antituberculous chemotherapy (IN/Rx). (B) NF-κB expression in BAL cells. Lanes 1–10 are the same blots as in A, lanes 2–11. Lanes 1 and 2, The level of NF-κB expression is similar in adherent (Ad) and nonadherent (Non) cells. Lanes 3–10, There is equal or increased NF-κB expression in the involved lobes (IN) compared with the uninvolved lobes (UN).
Figure 8
C/EBPβ binding to the HIV-1 LTR NRE in BAL cells from six patients with pulmonary tuberculosis. Extracts from uninvolved lung of patients with pulmonary tuberculosis predominantly express C/EBPβ, whereas BAL cells from involved lung downregulate C/EBPβ and switch expression to another C/EBP family member. Lane 1, NRE binding of THP-1 macrophages infected with M. tuberculosis. Lanes 2–4, 10–13, 18, 20, and 22, NRE–protein complex in uninvolved lobes (UN) of HIV-1–infected and HIV-1–negative patients with pulmonary tuberculosis. Lanes 2, 10, 18, 20, and 22, BAL cell extract strongly binds the NRE. A nonspecific band is also expressed in BAL extracts (double arrow). Lanes 3 and 11, 100-fold excess unlabeled NRE probe disrupts both the specific and nonspecific complex. Lanes 4 and 12, 100-fold excess unlabeled mutant probe does not affect the specific complex. Lanes 5 and 13, Anti-C/EBPβ antibody supershifts >90% of the complex. Lanes 6, 14, 19, 21, 23, and 24, Tuberculosis-involved lobes (IN) lose the predominant band expressed in uninvolved lobes and switch expression to a faster migrating specific NRE–protein complex (arrow). Lanes 7 and 15, 100-fold excess unlabeled NRE disrupts the complex. Lanes 8 and 16, 100-fold excess unlabeled mutant probe does not disrupt the complex. Lanes 9 and 17, Anti-C/EBPβ antibody does not supershift the complex. Lane 24, Adherent cells (Ad) from an involved lobe strongly express specific and nonspecific NRE– protein complexes. Lane 25, Nonadherent cells (Non) from the same involved lobe have markedly reduced expression of the specific and nonspecific NRE–protein complexes.
Similar articles
- Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression.
Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Weiden M, et al. J Immunol. 2000 Aug 15;165(4):2028-39. doi: 10.4049/jimmunol.165.4.2028. J Immunol. 2000. PMID: 10925286 - [Molecular pathogenesis in tuberculosis complicated with AIDS].
Nakata K, Hoshino Y, Honda Y, Tanaka N, Hebisawa A, Weiden M. Nakata K, et al. Kekkaku. 2004 Nov;79(11):659-67. Kekkaku. 2004. PMID: 15729891 Review. Japanese. - [Tuberculosis in patients with acquired immune deficiency syndrome].
Nakata K, Honda Y, Tanaka N, Weiden M, Keicho N. Nakata K, et al. Kekkaku. 2000 Sep;75(9):547-56. Kekkaku. 2000. PMID: 11068371 Review. Japanese. - Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis.
Hoshino Y, Hoshino S, Gold JA, Raju B, Prabhakar S, Pine R, Rom WN, Nakata K, Weiden M. Hoshino Y, et al. J Infect Dis. 2007 May 1;195(9):1303-10. doi: 10.1086/513438. Epub 2007 Mar 19. J Infect Dis. 2007. PMID: 17396999
Cited by
- Early emergence and selection of a SIV-LTR C/EBP site variant in SIV-infected macaques that increases virus infectivity.
Ravimohan S, Gama L, Engle EL, Zink MC, Clements JE. Ravimohan S, et al. PLoS One. 2012;7(8):e42801. doi: 10.1371/journal.pone.0042801. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952612 Free PMC article. - Systematic review and meta-analysis of hepatitis C virus infection and HIV viral load: new insights into epidemiologic synergy.
Petersdorf N, Ross JM, Weiss HA, Barnabas RV, Wasserheit JN; HCV and HIV Transmission Working Group. Petersdorf N, et al. J Int AIDS Soc. 2016 Sep 19;19(1):20944. doi: 10.7448/IAS.19.1.20944. eCollection 2016. J Int AIDS Soc. 2016. PMID: 27649908 Free PMC article. Review. - Oral infectious diseases: a potential risk factor for HIV virus recrudescence?
González OA, Ebersole JL, Huang CB. González OA, et al. Oral Dis. 2009 Jul;15(5):313-27. doi: 10.1111/j.1601-0825.2009.01533.x. Epub 2009 Apr 2. Oral Dis. 2009. PMID: 19364391 Free PMC article. Review. - Multiple Inhibitory Factors Act in the Late Phase of HIV-1 Replication: a Systematic Review of the Literature.
Gélinas JF, Gill DR, Hyde SC. Gélinas JF, et al. Microbiol Mol Biol Rev. 2018 Jan 10;82(1):e00051-17. doi: 10.1128/MMBR.00051-17. Print 2018 Mar. Microbiol Mol Biol Rev. 2018. PMID: 29321222 Free PMC article. Review. - Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients.
Witwer KW, Watson AK, Blankson JN, Clements JE. Witwer KW, et al. Retrovirology. 2012 Jan 12;9:5. doi: 10.1186/1742-4690-9-5. Retrovirology. 2012. PMID: 22240256 Free PMC article.
References
- Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. M. tuberculosisenhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. - PubMed
- Itescu S, Simonelli PF, Winchester RJ, Ginsberg HS. Human immunodeficiency virus type 1 strains in the lungs of infected individuals evolve independently from those in the peripheral blood and are highly conserved in the C-terminal region of the envelope V3 loop. Proc Natl Acad Sci USA. 1994;91:11378–11382. - PMC - PubMed
- Israel-Biet D, Cadranel J, Beldjord K, Andrieu J, Jeffrey A, Even P. Tumor necrosis factor production in HIV-seropositive subjects: relationship with lung opportunistic infections and HIV expression in alveolar macrophages. J Immunol. 1991;147:490–494. - PubMed
- Sierra-Madero J, Toossi Z, Hom D, Finegan C, Hoenig E, Rich E. Relationship between load of virus in alveolar macrophages from human immunodeficiency virus type 1-infected persons, production of cytokines, and clinical status. J Infect Dis. 1994;169:18–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000096/RR/NCRR NIH HHS/United States
- MO1 RR00096/RR/NCRR NIH HHS/United States
- HL-51470/HL/NHLBI NIH HHS/United States
- HL-51494/HL/NHLBI NIH HHS/United States
- R01 AI037877/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials