Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes - PubMed (original) (raw)
Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes
M L Albert et al. J Exp Med. 1998.
Abstract
Dendritic cells, but not macrophages, efficiently phagocytose apoptotic cells and cross-present viral, tumor, and self-antigens to CD8(+) T cells. This in vitro pathway corresponds to the in vivo phenomena of cross-priming and cross-tolerance. Here, we demonstrate that phagocytosis of apoptotic cells is restricted to the immature stage of dendritic cell (DC) development, and that this process is accompanied by the expression of a unique profile of receptors, in particular the alphavbeta5 integrin and CD36. Upon maturation, these receptors and, in turn, the phagocytic capacity of DCs, are downmodulated. Macrophages engulf apoptotic cells more efficiently than DCs, and although they express many receptors that mediate this uptake, they lack the alphavbeta5 integrin. Furthermore, in contrast to DCs, macrophages fail to cross-present antigenic material contained within the engulfed apoptotic cells. Thus, DCs use unique pathways for the phagocytosis, processing, and presentation of antigen derived from apoptotic cells on class I major histocompatibility complex. We suggest that the alphavbeta5 integrin plays a critical role in the trafficking of exogenous antigen by immature DCs in this cross-priming pathway.
Figures
Figure 1
Immature but not mature DCs efficiently phagocytose apoptotic cells. Freshly isolated blood monocytes were infected with live influenza A, PR/8 (Spafas Inc., Storrs, CT), labeled with the PKH26-GL fluorescent cell linker compound (Sigma Biosciences), and incubated at 37°C for 6–8 h, allowing apoptosis to occur. Macrophages, immature DCs, and mature DCs were dyed with PKH67-GL and added to the culture wells containing the apoptotic monocytes at a ratio of 1:1. Cells were analyzed by FACScan® where double positive cells indicate uptake of the apoptotic cells by the various APCs (iii, vi, and ix). We used the various APCs alone to establish the proper settings (i, iv, and vii). Note that as the forward scatter of the APCs increased, the dying monocytes were excluded from the established region (ii, v, and viii). After 2 h, 80% of the macrophages, 50% of the immature DCs, and <10% of the mature DCs had engulfed the apoptotic monocytes (A). In an independent experiment, macrophages (squares), immature DCs (diamonds), and mature DCs (circles) were prepared, and cocultures with apoptotic monocytes were established as described above. FACS® was performed at various time points. Percentage of phagocytosis was calculated based on the number of double positive cells (B).
Figure 1
Immature but not mature DCs efficiently phagocytose apoptotic cells. Freshly isolated blood monocytes were infected with live influenza A, PR/8 (Spafas Inc., Storrs, CT), labeled with the PKH26-GL fluorescent cell linker compound (Sigma Biosciences), and incubated at 37°C for 6–8 h, allowing apoptosis to occur. Macrophages, immature DCs, and mature DCs were dyed with PKH67-GL and added to the culture wells containing the apoptotic monocytes at a ratio of 1:1. Cells were analyzed by FACScan® where double positive cells indicate uptake of the apoptotic cells by the various APCs (iii, vi, and ix). We used the various APCs alone to establish the proper settings (i, iv, and vii). Note that as the forward scatter of the APCs increased, the dying monocytes were excluded from the established region (ii, v, and viii). After 2 h, 80% of the macrophages, 50% of the immature DCs, and <10% of the mature DCs had engulfed the apoptotic monocytes (A). In an independent experiment, macrophages (squares), immature DCs (diamonds), and mature DCs (circles) were prepared, and cocultures with apoptotic monocytes were established as described above. FACS® was performed at various time points. Percentage of phagocytosis was calculated based on the number of double positive cells (B).
Figure 7
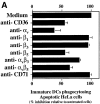
Direct inhibition of phagocytosis by anti-αvβ5 and anti-CD36 antibodies. HeLa cells were labeled with PKH26-GL, followed by irradiation using a 60 UVB lamp (Derma Control Inc.), calibrated to provide 240 mJ cm−2 in 2 min, sufficient for the induction of apoptosis. After 6–8 h, immature DCs dyed with PKH67-GL and pretreated with 50 μg/ml of various mAbs for 30 min were added to the wells containing apoptotic HeLa cells. 45–60 min later, cells were analyzed by FACS® for double positive cells. Phagocytic uptake is reported as a percentage of untreated cells. Maximal phagocytosis ranged from 44 to 52%. Results from three experiments were averaged and means plotted + SD. Similar results (data not shown) were obtained when apoptotic monocytes were used (A). Immature DCs were incubated with red fluorescent latex beads at 37°C (squares), 4°C (diamonds), or 37°C in the presence of 50 μg/ml anti-αvβ5 (circles), or anti-αv (triangles). The results shown indicate percentage of phagocytosis over background (B).
Figure 7
Direct inhibition of phagocytosis by anti-αvβ5 and anti-CD36 antibodies. HeLa cells were labeled with PKH26-GL, followed by irradiation using a 60 UVB lamp (Derma Control Inc.), calibrated to provide 240 mJ cm−2 in 2 min, sufficient for the induction of apoptosis. After 6–8 h, immature DCs dyed with PKH67-GL and pretreated with 50 μg/ml of various mAbs for 30 min were added to the wells containing apoptotic HeLa cells. 45–60 min later, cells were analyzed by FACS® for double positive cells. Phagocytic uptake is reported as a percentage of untreated cells. Maximal phagocytosis ranged from 44 to 52%. Results from three experiments were averaged and means plotted + SD. Similar results (data not shown) were obtained when apoptotic monocytes were used (A). Immature DCs were incubated with red fluorescent latex beads at 37°C (squares), 4°C (diamonds), or 37°C in the presence of 50 μg/ml anti-αvβ5 (circles), or anti-αv (triangles). The results shown indicate percentage of phagocytosis over background (B).
Figure 2
Low temperature, Cytochalasin D, and EDTA block phagocytosis of apoptotic cells by immature DCs. Apoptotic monocytes and immature DCs were prepared as described above. Immature DCs were preincubated at 4°C (A) in the presence of varying concentrations of Cytochalasin D (B) or EDTA (C) for 30 min. Apoptotic monocytes were then added to the DC cultures at 4°C (A) or 37°C (B and C). FACS® analysis was performed after 1–2 h. Data shown are representative of five independent experiments in which influenza- infected monocytes or UVB-irradiated HeLa cells were sources of apoptotic food for the immature DCs. Percentages of inhibition ± SD for these experiments were: 4°C, 85 ± 7%; 10 μM Cytochalasin D, 69 ± 3%; and 2 mM EDTA, 76 ± 14%.
Figure 3
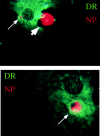
Immature DCs engulf influenza-infected monocytes. Influenza-infected apoptotic monocytes were cocultured with immature DCs for 1 h, after which the cells were adhered to a coverslip and fixed with acetone. Immunofluorescence was performed with antiinfluenza nucleoprotein antibodies (NP) and Texas red–conjugated goat anti–mouse IgG and by biotinylated anti–HLA-DR (DR) followed by FITC-conjugated streptavidin. The large arrowhead indicates apoptotic cell outside the DC before engulfment. Small arrows indicate apoptotic material derived from the influenza-infected monocytes within DR+ vesicles of the DC. These images were not generated on a confocal scope, so the structures of the DC underlying the apoptotic cell can be seen.
Figure 4
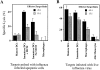
Immature DCs but not mature DCs or macrophages cross-present antigenic material derived from apoptotic cells. Various populations of HLA-A2.1+ APCs were cocultured with HLA-A2.1− influenza-infected monocytes. After 12 h, the APCs were loaded with 51Cr and used as targets for HLA-A2.1+ influenza-reactive CTL lines. Mature DCs were isolated by labeling with the DC-restricted marker CD83, followed by cell sorting on the FACSort®. Immature DCs were CD14− and were sorted by FACSort® as a CD83− population. Mature macrophages were generated by culturing an adherent mononuclear cell fraction in a Teflon beaker for 9 d. Effector/target ratios = 45:1 and 15:1 (A). Controls included infected and uninfected mature DCs, immature DCs, and macrophages. The HLA-A2.1− monocytes used as a source of apoptotic material were also tested as targets to demonstrate the absence of lysis when using a mismatched target. Effector/target ratios = 45:1 and 15:1. Results are representative of three experiments and the values shown represent the mean of triplicate wells (B).
Figure 5
Intracellular but not extracellular CD83 expression distinguishes immature DCs from mature DCs and macrophages. Macrophages, immature DCs, and mature DCs were prepared as previously described. Cells were either untreated (Surface) or permeabilized with saponin (Intracellular), and incubated with anti-CD83. Cells were then labeled using a PE-conjugated GAM-Ig (Sigma Biosciences). An isotype-matched antibody was used (IgG2a) as a control.
Figure 6
Protein and mRNA expression of αvβ5 and CD36 are downregulated during DC maturation. Immature DCs (A) and mature DCs (B) were incubated with anti-αvβ3 (clone 23C6; PharMingen), anti-CD36 (clone FA6; obtained from the fifth international workshop on leukocyte differentiation antigens), or anti-αvβ5 (clone P1F6; Chemicon International, Inc.), followed by PE-conjugated GAM-Ig (Sigma Biosciences). All cells were analyzed by FACScan®. (C) RNA was isolated from highly purified sorted cell populations of immature and mature DCs as previously described. Reverse transcription was carried out and after 30 cycles of PCR the distinct bands for β3, β5, and CD36 could be seen in the immature DCs (lane 1). In mature DCs, only a faint band for CD36 and no band for β5 could be visualized (lane 2), indicating minimal mRNA. In contrast, a band was evident for β3 in the mature DCs. Note that the doublet for β3 is an artifact in this particular exposure and does not indicate two unique bands (lane 2). As a positive control, extracts from Bowes melanoma cells, which are known to express β3 and CD36 (29), were run (lane 3). As negative control, the Bowes melanoma cells were run in the absence of a reverse transcriptase (lane 4).
Figure 6
Protein and mRNA expression of αvβ5 and CD36 are downregulated during DC maturation. Immature DCs (A) and mature DCs (B) were incubated with anti-αvβ3 (clone 23C6; PharMingen), anti-CD36 (clone FA6; obtained from the fifth international workshop on leukocyte differentiation antigens), or anti-αvβ5 (clone P1F6; Chemicon International, Inc.), followed by PE-conjugated GAM-Ig (Sigma Biosciences). All cells were analyzed by FACScan®. (C) RNA was isolated from highly purified sorted cell populations of immature and mature DCs as previously described. Reverse transcription was carried out and after 30 cycles of PCR the distinct bands for β3, β5, and CD36 could be seen in the immature DCs (lane 1). In mature DCs, only a faint band for CD36 and no band for β5 could be visualized (lane 2), indicating minimal mRNA. In contrast, a band was evident for β3 in the mature DCs. Note that the doublet for β3 is an artifact in this particular exposure and does not indicate two unique bands (lane 2). As a positive control, extracts from Bowes melanoma cells, which are known to express β3 and CD36 (29), were run (lane 3). As negative control, the Bowes melanoma cells were run in the absence of a reverse transcriptase (lane 4).
Similar articles
- CD36 or alphavbeta3 and alphavbeta5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8 alpha+ murine dendritic cells.
Schulz O, Pennington DJ, Hodivala-Dilke K, Febbraio M, Reis e Sousa C. Schulz O, et al. J Immunol. 2002 Jun 15;168(12):6057-65. doi: 10.4049/jimmunol.168.12.6057. J Immunol. 2002. PMID: 12055214 - Autophagy protein ATG5 regulates CD36 expression and anti-tumor MHC class II antigen presentation in dendritic cells.
Oh DS, Lee HK. Oh DS, et al. Autophagy. 2019 Dec;15(12):2091-2106. doi: 10.1080/15548627.2019.1596493. Epub 2019 Apr 6. Autophagy. 2019. PMID: 30900506 Free PMC article. - Delayed clearance of apoptotic lymphoma cells allows cross-presentation of intracellular antigens by mature dendritic cells.
Rovere P, Sabbadini MG, Vallinoto C, Fascio U, Zimmermann VS, Bondanza A, Ricciardi-Castagnoli P, Manfredi AA. Rovere P, et al. J Leukoc Biol. 1999 Aug;66(2):345-9. doi: 10.1002/jlb.66.2.345. J Leukoc Biol. 1999. PMID: 10449179 - Cross-presentation: dendritic cells and macrophages bite off more than they can chew!
Brode S, Macary PA. Brode S, et al. Immunology. 2004 Jul;112(3):345-51. doi: 10.1111/j.1365-2567.2004.01920.x. Immunology. 2004. PMID: 15196201 Free PMC article. Review. - The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells.
Singh-Jasuja H, Hilf N, Scherer HU, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. Singh-Jasuja H, et al. Cell Stress Chaperones. 2000 Nov;5(5):462-70. doi: 10.1379/1466-1268(2000)005<0462:thspga>2.0.co;2. Cell Stress Chaperones. 2000. PMID: 11189453 Free PMC article. Review.
Cited by
- αvβ5 integrin-dependent diurnal phagocytosis of shed photoreceptor outer segments by RPE cells is independent of the integrin coreceptor transglutaminase-2.
Ruggiero L, Sarang Z, Szondy Z, Finnemann SC. Ruggiero L, et al. Adv Exp Med Biol. 2012;723:731-7. doi: 10.1007/978-1-4614-0631-0_93. Adv Exp Med Biol. 2012. PMID: 22183400 Free PMC article. No abstract available. - A paradox of immunodeficiency and inflammation in human aging: lessons learned from apoptosis.
Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. Gupta S, et al. Immun Ageing. 2006 May 19;3:5. doi: 10.1186/1742-4933-3-5. Immun Ageing. 2006. PMID: 16712718 Free PMC article. - Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins.
Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Lacy-Hulbert A, et al. Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15823-8. doi: 10.1073/pnas.0707421104. Epub 2007 Sep 25. Proc Natl Acad Sci U S A. 2007. PMID: 17895374 Free PMC article. - Functional roles of immature dendritic cells in impaired immunity of solid tumour and their targeted strategies for provoking tumour immunity.
Kim R, Emi M, Tanabe K. Kim R, et al. Clin Exp Immunol. 2006 Nov;146(2):189-96. doi: 10.1111/j.1365-2249.2006.03215.x. Clin Exp Immunol. 2006. PMID: 17034569 Free PMC article. Review. - Immunoregulation of dendritic cells.
Wallet MA, Sen P, Tisch R. Wallet MA, et al. Clin Med Res. 2005 Aug;3(3):166-75. doi: 10.3121/cmr.3.3.166. Clin Med Res. 2005. PMID: 16160071 Free PMC article. Review.
References
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. - PubMed
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. - PubMed
- Savill J. Phagocytic docking without shocking. Nature. 1998;392:442–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials