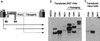A third-generation lentivirus vector with a conditional packaging system - PubMed (original) (raw)
A third-generation lentivirus vector with a conditional packaging system
T Dull et al. J Virol. 1998 Nov.
Abstract
Vectors derived from human immunodeficiency virus (HIV) are highly efficient vehicles for in vivo gene delivery. However, their biosafety is of major concern. Here we exploit the complexity of the HIV genome to provide lentivirus vectors with novel biosafety features. In addition to the structural genes, HIV contains two regulatory genes, tat and rev, that are essential for HIV replication, and four accessory genes that encode critical virulence factors. We previously reported that the HIV type 1 accessory open reading frames are dispensable for efficient gene transduction by a lentivirus vector. We now demonstrate that the requirement for the tat gene can be offset by placing constitutive promoters upstream of the vector transcript. Vectors generated from constructs containing such a chimeric long terminal repeat (LTR) transduced neurons in vivo at very high efficiency, whether or not they were produced in the presence of Tat. When the rev gene was also deleted from the packaging construct, expression of gag and pol was strictly dependent on Rev complementation in trans. By the combined use of a separate nonoverlapping Rev expression plasmid and a 5' LTR chimeric transfer construct, we achieved optimal yields of vector of high transducing efficiency (up to 10(7) transducing units [TU]/ml and 10(4) TU/ng of p24). This third-generation lentivirus vector uses only a fractional set of HIV genes: gag, pol, and rev. Moreover, the HIV-derived constructs, and any recombinant between them, are contingent on upstream elements and trans complementation for expression and thus are nonfunctional outside of the vector producer cells. This split-genome, conditional packaging system is based on existing viral sequences and acts as a built-in device against the generation of productive recombinants. While the actual biosafety of the vector will ultimately be proven in vivo, the improved design presented here should facilitate testing of lentivirus vectors.
Figures
FIG. 1
Northern analysis of the RNA expression from lentivirus vectors. Three pHR2 vectors carrying an expression cassette for the same transgene (truncated low-affinity NGFR) and driven by three different promoters (PGK, CMV, and retroviral MFG) were analyzed in producer and transduced cells. Total RNA was extracted and analyzed by Northern blotting with a probe specific for the transgene sequence. (A) Schematic of the vector construct depicts the species of RNA driven by the internal promoter (Prom.; broken arrow, shorter transcript) and the viral LTR (solid arrows, longer transcripts; the two species differ for the splicing of the viral intron). The splice donor and acceptor sites (SD and SA), the packaging sequence (Ψ), the truncated gag sequence (GA), and the RRE are indicated. (B) The vector constructs were transfected in 293T cells without or with the packaging construct. (C) Vector particles produced by the 293T transfectants were used to transduce HeLa cells. In the absence of the viral transactivators, supplied by the core packaging construct only in the producer cells, vector expression occurs mainly from the internal promoter. Note the dramatic enhancement of the upstream transcription and the accumulation of unspliced RNA (carrying the Ψ sequence) in the presence of the packaging construct. In the transduced cells, the LTR is silenced. Note that the three expression cassettes differ in the size of the promoters and 5′ untranslated sequence. In each case, the smallest RNA species represents transcripts initiated from the internal promoter, while the intermediate-size and larger species correspond to spliced and unspliced LTR-driven RNAs, respectively.
FIG. 2
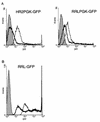
Transcriptional activities of wild-type and 5′ chimeric vector constructs in the absence and presence of Tat. (A) Control pHR2 and the 5′ chimeric pRRL transfer construct carrying a PGK-eGFP expression cassette were transfected into 293T cells with a packaging construct having a functional (pCMVΔR8.91; grey line) or inactive (pCMVΔR8.93; black line) tat gene. GFP expression was analyzed by FACS. The filled area represents nontransfected cells. In the absence of Tat, the chimeric construct yielded a level of GFP expression higher than that achieved by the pHR2 construct. Both constructs were further upregulated by Tat. (B) A pRRL construct carrying the eGFP gene without an internal promoter was transfected with a packaging construct carrying a functional (grey line, open area) or inactive (black line, open area) tat gene. Direct upregulation of the chimeric promoter by Tat was observed. The filled area represents nontransfected cells.
FIG. 3
In vivo transduction of eGFP into brain cells by lentivirus vectors produced with and without Tat. Vectors carrying a PGK-eGFP expression cassette were produced by the pHR2 (A and B) or the 5′ chimeric pRRL (C and D) transfer construct and a packaging construct with (pCMVΔR8.91; A and C) or without (pCMVΔR8.93; B and D) a functional tat gene, concentrated by ultracentrifugation, and normalized for particle content prior to injection into the corpora striata of adult rats. One month after injection, brain sections were stained for immunoreactivity to the GFP protein. While both types of vectors transduced neurons very efficiently when made with Tat, only the vector made by the chimeric transfer construct worked as well when produced without Tat. Representative sections close to the injection site are shown for one of six striata injected per each type of vector. The bar in panel B represents 1 mm; that in the inset in panel A represents 100 μm.
FIG. 4
Schematic drawing of the HIV provirus and the four constructs used to make a lentivirus vector of the third generation. The viral LTRs, the reading frames of the viral genes, the major 5′ splice donor site (SD), the packaging sequence (Ψ), and the RRE are boxed and indicated in bold type. The conditional packaging construct, pMDLg/pRRE, expresses the gag and pol genes from the CMV promoter and intervening sequences and polyadenylation site of the human β-globin gene. As the transcripts of the gag and pol genes contain _cis_-repressive sequences, they are expressed only if Rev promotes their nuclear export by binding to the RRE. All tat and rev exons have been deleted, and the viral sequences upstream of the gag gene have been replaced. A nonoverlapping construct, RSV-Rev, expresses the rev cDNA. The transfer construct, pRRL.SIN-18, contains HIV-1 _cis_-acting sequences and an expression cassette for the transgene. It is the only portion transferred to the target cells and does not contain wild-type copies of the HIV LTR. The 5′ LTR is chimeric, with the enhancer/promoter of RSV replacing the U3 region (RRL) to rescue the transcriptional dependence on Tat. The 3′ LTR has an almost complete deletion of the U3 region, which includes the TATA box (from nucleotides −418 to −18 relative to the U3/R border). As the latter is the template used to generate both copies of the LTR in the integrated provirus, transduction of this vector results in transcriptional inactivation of both LTRs; thus, it is a self-inactivating vector (SIN-18). The fourth construct, pMD.G, encodes a heterologous envelope to pseudotype the vector, here shown coding for VSV G. Only the relevant parts of the constructs are shown.
Similar articles
- A new-generation stable inducible packaging cell line for lentiviral vectors.
Farson D, Witt R, McGuinness R, Dull T, Kelly M, Song J, Radeke R, Bukovsky A, Consiglio A, Naldini L. Farson D, et al. Hum Gene Ther. 2001 May 20;12(8):981-97. doi: 10.1089/104303401750195935. Hum Gene Ther. 2001. PMID: 11387062 - Generation of a packaging cell line for prolonged large-scale production of high-titer HIV-1-based lentiviral vector.
Ni Y, Sun S, Oparaocha I, Humeau L, Davis B, Cohen R, Binder G, Chang YN, Slepushkin V, Dropulic B. Ni Y, et al. J Gene Med. 2005 Jun;7(6):818-34. doi: 10.1002/jgm.726. J Gene Med. 2005. PMID: 15693055 - Production of lentiviral vectors for transducing cells from the central nervous system.
Li M, Husic N, Lin Y, Snider BJ. Li M, et al. J Vis Exp. 2012 May 24;(63):e4031. doi: 10.3791/4031. J Vis Exp. 2012. PMID: 22664962 Free PMC article. - Safety considerations in vector development.
Kappes JC, Wu X. Kappes JC, et al. Somat Cell Mol Genet. 2001 Nov;26(1-6):147-58. doi: 10.1023/a:1021082815013. Somat Cell Mol Genet. 2001. PMID: 12465466 Review. - Lentiviral vectors for gene therapy of HIV-1 infection.
Mautino MR. Mautino MR. Curr Gene Ther. 2002 Feb;2(1):23-43. doi: 10.2174/1566523023348165. Curr Gene Ther. 2002. PMID: 12108972 Review.
Cited by
- In vivo imaging enables high resolution preclinical trials on patients' leukemia cells growing in mice.
Terziyska N, Castro Alves C, Groiss V, Schneider K, Farkasova K, Ogris M, Wagner E, Ehrhardt H, Brentjens RJ, zur Stadt U, Horstmann M, Quintanilla-Martinez L, Jeremias I. Terziyska N, et al. PLoS One. 2012;7(12):e52798. doi: 10.1371/journal.pone.0052798. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300782 Free PMC article. - The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy.
Webster CP, Smith EF, Bauer CS, Moller A, Hautbergue GM, Ferraiuolo L, Myszczynska MA, Higginbottom A, Walsh MJ, Whitworth AJ, Kaspar BK, Meyer K, Shaw PJ, Grierson AJ, De Vos KJ. Webster CP, et al. EMBO J. 2016 Aug 1;35(15):1656-76. doi: 10.15252/embj.201694401. Epub 2016 Jun 22. EMBO J. 2016. PMID: 27334615 Free PMC article. - The Hemogenic Competence of Endothelial Progenitors Is Restricted by Runx1 Silencing during Embryonic Development.
Eliades A, Wareing S, Marinopoulou E, Fadlullah MZH, Patel R, Grabarek JB, Plusa B, Lacaud G, Kouskoff V. Eliades A, et al. Cell Rep. 2016 Jun 7;15(10):2185-2199. doi: 10.1016/j.celrep.2016.05.001. Epub 2016 May 26. Cell Rep. 2016. PMID: 27239041 Free PMC article. - The dystonia gene THAP1 controls DNA double-strand break repair choice.
Shinoda K, Zong D, Callen E, Wu W, Dumitrache LC, Belinky F, Chari R, Wong N, Ishikawa M, Stanlie A, Multhaupt-Buell T, Sharma N, Ozelius L, Ehrlich M, McKinnon PJ, Nussenzweig A. Shinoda K, et al. Mol Cell. 2021 Jun 17;81(12):2611-2624.e10. doi: 10.1016/j.molcel.2021.03.034. Epub 2021 Apr 14. Mol Cell. 2021. PMID: 33857404 Free PMC article. - β-globin gene transfer to human bone marrow for sickle cell disease.
Romero Z, Urbinati F, Geiger S, Cooper AR, Wherley J, Kaufman ML, Hollis RP, de Assin RR, Senadheera S, Sahagian A, Jin X, Gellis A, Wang X, Gjertson D, Deoliveira S, Kempert P, Shupien S, Abdel-Azim H, Walters MC, Meiselman HJ, Wenby RB, Gruber T, Marder V, Coates TD, Kohn DB. Romero Z, et al. J Clin Invest. 2013 Jul 1;123(8):3317-30. doi: 10.1172/JCI67930. Online ahead of print. J Clin Invest. 2013. PMID: 23863630 Free PMC article.
References
- Berkowitz R D, Hammarskjöld M L, Helga-Maria C, Rekosh D, Goff S P. 5′ regions of HIV-1 RNAs are not sufficient for encapsidation: implications for the HIV-1 packaging signal. Virology. 1995;212:718–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials