The role of interferon in influenza virus tissue tropism - PubMed (original) (raw)
The role of interferon in influenza virus tissue tropism
A García-Sastre et al. J Virol. 1998 Nov.
Abstract
We have studied the pathogenesis of influenza virus infection in mice that are unable to respond to type I or II interferons due to a targeted disruption of the STAT1 gene. STAT1-/- animals are 100-fold more sensitive to lethal infection with influenza A/WSN/33 virus than are their wild-type (WT) counterparts. Virus replicated only in the lungs of WT animals following intranasal (i.n.) virus inoculation, while STAT1-/- mice developed a fulminant systemic influenza virus infection following either i.n. or intraperitoneal inoculation. We investigated the mechanism underlying this altered virus tropism by comparing levels of virus replication in fibroblast cell lines and murine embryonic fibroblasts derived from WT mice, STAT-/- mice, and mice lacking gamma interferon (IFNgamma-/- mice) or the IFN-alpha receptor (IFNalphaR-/- mice). Influenza A/WSN/33 virus replicates to high titers in STAT1-/- or IFNalphaR-/- fibroblasts, while cells derived from WT or IFNgamma-/- animals are resistant to influenza virus infection. Immunofluorescence studies using WT fibroblast cell lines demonstrated that only a small subpopulation of WT cells can be infected and that in the few infected WT cells, virus replication is aborted at an early, nuclear phase. In all organs examined except the lung, influenza A WSN/33 virus infection is apparently prevented by an intact type I interferon response. Our results demonstrate that type I interferon plays an important role in determining the pathogenicity and tissue restriction of influenza A/WSN/33 virus in vivo and in vitro.
Figures
FIG. 1
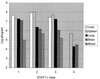
Determination of lung virus titers over a 9-day period for WT (■) and STAT1−/− (▵) CD1 mice inoculated i.n. with WSN and PR8 viruses. (A) Animals of each genotype were inoculated i.n. with 1,000 PFU (1 LD50 for a WT animal) of WSN virus in 50 μl of PBS. Four to five WT and STAT1−/− animals were sacrificed at each time point. Results from two experiments are included. Viral titers in lung homogenates from each animal (PFU/gram of tissue) were determined by plaque assay in MDCK cells. (B) Fifteen animals of each genotype were inoculated i.n. with 10 TCID (1 LD50 for a WT animal) of PR8 virus in 100 μl of PBS. Five WT and five STAT1−/− animals were sacrificed at days 3, 6, and 9 p.i. Viral titers in lung homogenates from each animal were determined as TCID/milliliter.
FIG. 2
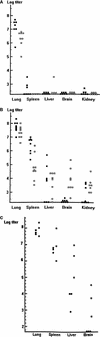
Virus titers following i.p. WSN virus inoculation of STAT1−/− mice. Four STAT1−/− and three WT mice were injected i.p. with 107 PFU of WSN virus. Animals were sacrificed at day 4 postinoculation when the mutant animals showed signs of illness. Viral titers of tissue homogenates were determined by plaque assay in MDCK cells and are expressed as PFU/gram of tissue. Virus titers for each tissue are shown for each of the STAT1−/− animals. No virus could be detected in samples obtained from three WT animals.
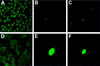
FIG. 3
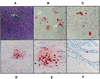
Pathology of hepatic and central nervous system lesions of STAT1−/− mice following i.p. injection with WSN virus. (A) Hematoxylin-eosin-stained section of liver showing multiple aggregates of inflammatory cells within the hepatic parenchyma. Magnification, ×98. (B [magnification, ×197] and C [magnification, ×394]) Sections reacted with polyclonal antibody against influenza A virus. Infected cells are bright red. Only a fraction of inflammatory cells constituting the liver lesions show positive staining for influenza virus antigens. Multiple hepatocytes stain strongly, some showing only nuclear staining, indicating an early phase of virus replication. In other hepatocytes, viral proteins are also present in the cytoplasm. (D) Section of brain with a focus staining positively for the presence of viral antigen (magnification, ×98). In panel E (magnification, ×394), it can be appreciated that many of the red-staining cells are neurons. Glial cells within the focus also stain positively. (F) Single infected ependymal cell (magnification, ×394).
FIG. 4
Comparison of tissue virus titers in WT (A), STAT1−/− (B), and IFNαR−/− (C) mice at different days after i.n. inoculation with WSN virus. (A and B) Eight to 10 WT (A) or STAT1−/− (B) mice were infected i.n. with 1,000 PFU of WSN virus, and viral titers in the indicated tissues at days 3 (■) and 6 (□) p.i. were determined by plaque assay in MDCK cells. Results from two experiments are expressed as PFU/gram of tissue. (C) Six IFNαR−/− mice were infected i.n. with 1,000 PFU of WSN virus, and viral titers at days 4 (■) and 8 (□) p.i. were determined by plaque assay in MDCK cells. Results are expressed as PFU/gram of tissue.
FIG. 5
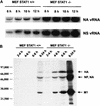
Viral RNA and protein expression levels in MEFs derived from WT (STAT+/+) and STAT−/− CD1 mice. (A) STAT+/+ and STAT−/− MEFs were infected with WSN virus at an MOI of 5, and vRNA levels specific for the NA and NS genes were determined by PAGE analysis of primer extension products at different times p.i. (B) STAT+/+ and STAT−/− MEFs were infected with WSN virus at an MOI of 2 and 35S labeled at the indicated time points, and total amount of viral proteins was immunoprecipitated with a polyclonal antiserum against WSN virus. Immunoprecipitated products were analyzed by SDS-PAGE.
FIG. 6
Immunofluorescence analysis of NP expression in WT and STAT1−/− MEFs infected with WSN virus. Cells were infected with WSN virus (MOI = 2) and stained with a monoclonal antibody against NP 14 h p.i. (A and D) Different-magnification fields of STAT1−/− cells. The majority of the cells showed a cytoplasmic NP staining, indicative of a late phase of virus replication. (B and C) Low magnification of two different fields of WT-infected cells. Although cell densities were roughly similar between the WT and STAT1−/− samples, only a few WT cells showed positive NP staining. (E and F) Higher magnification of individual positive-stained WT cells. Note that the majority of the NP staining is nuclear, which indicates a delayed or abortive viral replication.
Similar articles
- The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice.
Price GE, Gaszewska-Mastarlarz A, Moskophidis D. Price GE, et al. J Virol. 2000 May;74(9):3996-4003. doi: 10.1128/jvi.74.9.3996-4003.2000. J Virol. 2000. PMID: 10756011 Free PMC article. - The alpha/beta interferon receptor provides protection against influenza virus replication but is dispensable for inflammatory response signaling.
Goodman AG, Zeng H, Proll SC, Peng X, Cillóniz C, Carter VS, Korth MJ, Tumpey TM, Katze MG. Goodman AG, et al. J Virol. 2010 Feb;84(4):2027-37. doi: 10.1128/JVI.01595-09. Epub 2009 Nov 25. J Virol. 2010. PMID: 19939913 Free PMC article. - Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system.
Aronsson F, Robertson B, Ljunggren HG, Kristensson K. Aronsson F, et al. Viral Immunol. 2003;16(3):415-23. doi: 10.1089/088282403322396208. Viral Immunol. 2003. PMID: 14583155 - Dissemination of influenza B virus to the lower respiratory tract of mice is restricted by the interferon response.
Schwab LSU, Do THT, Pilapitiya D, Koutsakos M. Schwab LSU, et al. J Virol. 2024 Jun 13;98(6):e0160423. doi: 10.1128/jvi.01604-23. Epub 2024 May 23. J Virol. 2024. PMID: 38780249 - Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems.
García-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. García-Sastre A, et al. Virology. 1998 Dec 20;252(2):324-30. doi: 10.1006/viro.1998.9508. Virology. 1998. PMID: 9878611
Cited by
- Detection and characterization of H5N1 HPAIV in environmental samples from a dairy farm.
Singh G, Trujillo JD, McDowell CD, Matias-Ferreyra F, Kafle S, Kwon T, Gaudreault NN, Fitz I, Noll L, Morozov I, Retallick J, Richt JA. Singh G, et al. Virus Genes. 2024 Oct;60(5):517-527. doi: 10.1007/s11262-024-02085-4. Epub 2024 Jul 15. Virus Genes. 2024. PMID: 39008139 - STAT1 regulates neutrophil gelatinase B-associated lipocalin induction in influenza-induced myocarditis.
Constantinesco NJ, Srikanth S, De Vito L, Moras C, Ramasubramanian V, Chinnappan B, Hartwick S, Schwab KE, Wu Y, Gopal R. Constantinesco NJ, et al. Sci Rep. 2024 May 15;14(1):11124. doi: 10.1038/s41598-024-61953-z. Sci Rep. 2024. PMID: 38750107 Free PMC article. - CNS Viral Infections-What to Consider for Improving Drug Treatment: A Plea for Using Mathematical Modeling Approaches.
Sun M, Manson ML, Guo T, de Lange ECM. Sun M, et al. CNS Drugs. 2024 May;38(5):349-373. doi: 10.1007/s40263-024-01082-3. Epub 2024 Apr 5. CNS Drugs. 2024. PMID: 38580795 Free PMC article. Review. - Aromatic amino acid metabolites alter interferon signaling and influenza pathogenesis.
Anand G, Clark-Dinovo C, Perry AM, Goodwin VM, St Raymond E, Sakleshpur S, Steed AL. Anand G, et al. Front Mol Biosci. 2024 Jan 23;10:1232573. doi: 10.3389/fmolb.2023.1232573. eCollection 2023. Front Mol Biosci. 2024. PMID: 38322710 Free PMC article. - Exposome in ischaemic heart disease: beyond traditional risk factors.
Montone RA, Camilli M, Calvieri C, Magnani G, Bonanni A, Bhatt DL, Rajagopalan S, Crea F, Niccoli G. Montone RA, et al. Eur Heart J. 2024 Feb 7;45(6):419-438. doi: 10.1093/eurheartj/ehae001. Eur Heart J. 2024. PMID: 38238478 Free PMC article. Review.
References
- Anonymous. Isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, May–December 1997. Morbid Mortal Weekly Rep. 1997;46:1204–1207. - PubMed
- Bosch F X, Garten W, Klenk H-D, Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza viruses. Virology. 1981;113:725–735. - PubMed
- Boycott R, Klenk H-D, Ohuchi M. Cell tropism of influenza virus mediated by hemagglutinin activation at the stage of virus entry. Virology. 1994;203:313–319. - PubMed
- Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous