Human cytotoxic T-lymphocyte repertoire to influenza A viruses - PubMed (original) (raw)
Human cytotoxic T-lymphocyte repertoire to influenza A viruses
J Jameson et al. J Virol. 1998 Nov.
Abstract
The murine CD8(+) cytotoxic-T-lymphocyte (CTL) repertoire appears to be quite limited in response to influenza A viruses. The CTL responses to influenza A virus in humans were examined to determine if the CTL repertoire is also very limited. Bulk cultures revealed that a number of virus proteins were recognized in CTL assays. CTL lines were isolated from three donors for detailed study and found to be specific for epitopes on numerous influenza A viral proteins. Eight distinct CD8(+) CTL lines were isolated from donor 1. The proteins recognized by these cell lines included the nucleoprotein (NP), matrix protein (M1), nonstructural protein 1 (NS1), polymerases (PB1 and PB2), and hemagglutinin (HA). Two CD4(+) cell lines, one specific for neuraminidase (NA) and the other specific for M1, were also characterized. These CTL results were confirmed by precursor frequency analysis of peptide-specific gamma interferon-producing cells detected by ELISPOT. The epitopes recognized by 6 of these 10 cell lines have not been previously described; 8 of the 10 cell lines were cross-reactive to subtype H1N1, H2N2, and H3N2 viruses, 1 cell line was cross-reactive to subtypes H1N1 and H2N2, and 1 cell line was subtype H1N1 specific. A broad CTL repertoire was detected in the two other donors, and cell lines specific for the NP, NA, HA, M1, NS1, and M2 viral proteins were isolated. These findings indicate that the human memory CTL response to influenza A virus is broadly directed to epitopes on a wide variety of proteins, unlike the limited response observed following infection of mice.
Figures
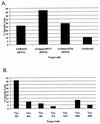
FIG. 1
(A) Influenza A virus subtype-cross-reactive lysis in bulk culture (donor 1). PBMC were stimulated with A/PR/8/34 virus in vitro on days 0 and 7 and tested on day 14 at an E-T ratio of 60:1. (B) Recognition of influenza A virus proteins in a bulk culture CTL assay (donor 1). The same bulk culture was tested for specific lysis of influenza viral proteins expressed in vaccinia virus (Vac.) constructs infected in BLCL at an E-T ratio of 60:1. Lysis of targets infected with wild-type vaccinia virus was subtracted from lysis of recombinant vaccinia virus-infected targets.
FIG. 2
MHC restriction of cell lines determined by using panels of partially HLA-matched allogeneic targets infected with vaccinia virus recombinants expressing either an influenza virus protein or a specific peptide. Lysis of targets infected with wild-type vaccinia virus was subtracted from lysis of recombinant vaccinia virus-infected targets. (A) Bulk culture cells tested against targets loaded with the NP aa 174 to 184 peptide. (B) Cell line 10-1B7 tested against vaccinia virus PB2-infected targets. (C) Cell line 1-2F8 tested against vaccinia virus PB1-infected targets. (D) Cell line 4-30E11 tested against vaccinia virus M1-infected targets. (E) Cell line 10-1G5 tested against vaccinia virus HA-infected targets. The E-T ratio was 10:1, except for panel A, for which the E-T ratio was 30:1.
FIG. 3
Mapping of CTL epitopes by using peptides. The epitopes recognized by cell line 10-1C4 from donor 1 (A), 3E5 from donor 2 (B), and 124 (C) and 77 (D) from donor 3 were mapped by using peptide-pulsed BLCL targets at a concentration of 25 μg/ml. The E-T ratios were 10:1 (A and B), 7.5:1 (C), and 15:1 (D).
FIG. 4
Dose response of peptide-pulsed target cell lysis. Cell line 10-1C4 from donor 1 (A), 3E5 from donor 2 (B), and 124 (C) and 77 (D) from donor 3 recognized target cells pulsed with various peptide concentrations. The E-T ratios were 10:1 (A and B) and 15:1 (C and D).
Similar articles
- Human memory CTL response specific for influenza A virus is broad and multispecific.
Gianfrani C, Oseroff C, Sidney J, Chesnut RW, Sette A. Gianfrani C, et al. Hum Immunol. 2000 May;61(5):438-52. doi: 10.1016/s0198-8859(00)00105-1. Hum Immunol. 2000. PMID: 10773346 - Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: identification of virus-specific and cross-reactive CD8(+) CTL epitopes on nucleocapsid protein.
Van Epps HL, Schmaljohn CS, Ennis FA. Van Epps HL, et al. J Virol. 1999 Jul;73(7):5301-8. doi: 10.1128/JVI.73.7.5301-5308.1999. J Virol. 1999. PMID: 10364276 Free PMC article. - Recognition of the PB1, neuraminidase, and matrix proteins of influenza virus A/NT/60/68 by cytotoxic T lymphocytes.
Reay PA, Jones IM, Gotch FM, McMichael AJ, Brownlee GG. Reay PA, et al. Virology. 1989 Jun;170(2):477-85. doi: 10.1016/0042-6822(89)90439-x. Virology. 1989. PMID: 2658303 - The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype.
Boon AC, de Mutsert G, Graus YM, Fouchier RA, Sintnicolaas K, Osterhaus AD, Rimmelzwaan GF. Boon AC, et al. J Virol. 2002 Jan;76(2):582-90. doi: 10.1128/jvi.76.2.582-590.2002. J Virol. 2002. PMID: 11752149 Free PMC article. - Cross-reactive human B cell and T cell epitopes between influenza A and B viruses.
Terajima M, Babon JA, Co MD, Ennis FA. Terajima M, et al. Virol J. 2013 Jul 26;10:244. doi: 10.1186/1743-422X-10-244. Virol J. 2013. PMID: 23886073 Free PMC article. Review.
Cited by
- The frequency and function of nucleoprotein-specific CD8+ T cells are critical for heterosubtypic immunity against influenza virus infection.
Amoah S, Cao W, Sayedahmed EE, Wang Y, Kumar A, Mishina M, Eddins DJ, Wang W-C, Burroughs M, Sheth M, Lee J, Shieh W-J, Ray SD, Bohannon CD, Ranjan P, Sharma SD, Hoehner J, Arthur RA, Gangappa S, Wakamatsu N, Johnston HR, Pohl J, Mittal SK, Sambhara S. Amoah S, et al. J Virol. 2024 Aug 20;98(8):e0071124. doi: 10.1128/jvi.00711-24. Epub 2024 Jul 31. J Virol. 2024. PMID: 39082839 Free PMC article. - BCG immunization induces CX3CR1hi effector memory T cells to provide cross-protection via IFN-γ-mediated trained immunity.
Tran KA, Pernet E, Sadeghi M, Downey J, Chronopoulos J, Lapshina E, Tsai O, Kaufmann E, Ding J, Divangahi M. Tran KA, et al. Nat Immunol. 2024 Mar;25(3):418-431. doi: 10.1038/s41590-023-01739-z. Epub 2024 Jan 15. Nat Immunol. 2024. PMID: 38225437 - A Multicenter, Controlled Human Infection Study of Influenza A(H1N1)pdm09 in Healthy Adults.
Ortiz JR, Bernstein DI, Hoft DF, Woods CW, McClain MT, Frey SE, Brady RC, Bryant C, Wegel A, Frenck RW, Walter EB, Abate G, Williams SR, Atmar RL, Keitel WA, Rouphael N, Memoli MJ, Makhene MK, Roberts PC, Neuzil KM. Ortiz JR, et al. J Infect Dis. 2023 Aug 11;228(3):287-298. doi: 10.1093/infdis/jiad021. J Infect Dis. 2023. PMID: 36702771 Free PMC article. - The Role of Nucleoprotein in Immunity to Human Negative-Stranded RNA Viruses-Not Just Another Brick in the Viral Nucleocapsid.
Šantak M, Matić Z. Šantak M, et al. Viruses. 2022 Mar 3;14(3):521. doi: 10.3390/v14030521. Viruses. 2022. PMID: 35336928 Free PMC article. Review. - Evolutionary pressures rendered by animal husbandry practices for avian influenza viruses to adapt to humans.
Martins de Camargo M, Caetano AR, Ferreira de Miranda Santos IK. Martins de Camargo M, et al. iScience. 2022 Mar 1;25(4):104005. doi: 10.1016/j.isci.2022.104005. eCollection 2022 Apr 15. iScience. 2022. PMID: 35313691 Free PMC article. Review.
References
- Bennink J R, Yewdell J W, Gerhard W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature. 1982;296:75–76. - PubMed
- Boyam A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;21:77–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous