Rhesus macaques infected with macrophage-tropic simian immunodeficiency virus (SIVmacR71/17E) exhibit extensive focal segmental and global glomerulosclerosis - PubMed (original) (raw)
Rhesus macaques infected with macrophage-tropic simian immunodeficiency virus (SIVmacR71/17E) exhibit extensive focal segmental and global glomerulosclerosis
E B Stephens et al. J Virol. 1998 Nov.
Abstract
We previously showed that inoculation of rhesus macaques with molecularly cloned lymphocytetropic simian immunodeficiency virus (SIVmac239) results in SIV-associated nephropathy (SIVAN) and that the glomerulosclerotic lesions were associated with the selection of macrophagetropic (M-tropic) variants (V. H. Gattone et al., AIDS Res. Hum. Retroviruses 14:1163-1180, 1998). In the present study, seven rhesus macaques were inoculated with M-tropic SIVmacR71/17E, and the renal pathology was examined at necropsy. All SIVmacR71/17E-infected macaques developed AIDS, and most developed other systemic complications, including SIV-induced encephalitis and lentivirus interstitial pneumonia. There was no correlation between the length of infection (42 to 97 days), circulating CD4(+) T-cell counts, and renal disease. Of the seven macaques inoculated with SIVmacR71/17E, five developed significant mesangial hyperplasia and expansion of matrix and four were clearly azotemic (serum urea nitrogen concentration of 40 to 112 mg/dl). These same five macaques developed focal segmental to global glomerulosclerotic lesions. Increased numbers of glomerular CD68(+) cells (monocytes/macrophages) were found in glomeruli but not the tubulointerstitium of the macaques inoculated with SIVmacR71/17E. All macaques had glomerular deposits of immunoglobulin G (IgG), IgM, and tubuloreticular inclusions, and six of seven had IgA deposition. However, there was no correlation between the presence of circulating anti-SIVmac antibodies, immunoglobulin deposition, and glomerular disease. Tubulointerstitial infiltrates were mild, with little or no correlation to azotemia, while microcystic tubules were evident in those with glomerulosclerosis or azotemia. The four most severely affected macaques were positive for diffuse glomerular immunostaining for viral core p27 antigen, and there was intense staining in the glomeruli of the two macaques with the most severe glomerulosclerosis. Viral sequences were isolated from glomerular and tubulointerstitial fractions from macaques with severe glomerulosclerosis but only from the tubulointerstitial compartment of those that did not develop glomerulosclerosis. Interviral recombinant viruses generated with env sequences isolated from glomeruli confirmed the M-tropic nature of the virus found in the glomeruli. The correlation between the increased number of CD68(+) cells (monocytes/macrophages) in the glomeruli, the localization of p27 antigen in the glomeruli, and the glomerular pathology confirms and extends our previous observations of an association between glomerular infection and infiltration by M-tropic virus and SIVAN.
Figures
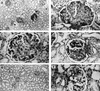
FIG. 1
Renal histopathology in macaques infected with SIVmacR71/17E. (a) Light micrograph of AQ20 (a group 1 macaque) with a focus of inflammatory cell infiltrate (arrowhead), slightly dilated tubules with cast material (arrows), and glomeruli (g), many of which are sclerotic (magnification, ×25). (b) A globally sclerotic glomerulus from AQ20 in which mesangial matrix has completely replaced the glomerular capillaries (magnification, ×100). (c) A sclerotic glomerulus from AQ47 with peripheral glomerular capillary collapse and sclerosis associated with the core of each lobule (magnification, ×100). (d) A glomerulus from AQ70 (a group 1 macaque) exhibiting FSGS. The top lobe of the glomerulus has numerous capillaries, while much of the bottom lobe is composed of mesangial matrix (magnification, ×100). (e and f) Light micrographs of AQ43 (a group 2 macaque) with normal-appearing kidney parenchyma and glomeruli (magnifications, ×25 and ×100).
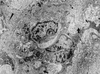
FIG. 2
Electron microscopy of glomerular pathology in macaques infected with SIVmacR71/17E. (a) Transmission electron micrograph of a portion of a glomerulus from AQ20 (a group 1 macaque) with prominent mesangial cells (M) surrounded by excessive amounts of extracellular matrix including both fibrillar (large arrows) and fibrous (small arrows) collagenous material. Within some of the fibrillar matrix are foci of amorphous material which has an increased electron density consistent with deposits of immunoglobulin (arrowheads) (magnification, ×7,600). (b) Transmission electron micrograph of a glomerular capillary loop from AQ43 (a group 2 macaque) showing a paracrystalloid tubuloreticular inclusion (curved arrow) within the glomerular endothelial cells. These inclusions were evident in all SIV-infected macaques (magnification, ×3,800).
FIG. 2
Electron microscopy of glomerular pathology in macaques infected with SIVmacR71/17E. (a) Transmission electron micrograph of a portion of a glomerulus from AQ20 (a group 1 macaque) with prominent mesangial cells (M) surrounded by excessive amounts of extracellular matrix including both fibrillar (large arrows) and fibrous (small arrows) collagenous material. Within some of the fibrillar matrix are foci of amorphous material which has an increased electron density consistent with deposits of immunoglobulin (arrowheads) (magnification, ×7,600). (b) Transmission electron micrograph of a glomerular capillary loop from AQ43 (a group 2 macaque) showing a paracrystalloid tubuloreticular inclusion (curved arrow) within the glomerular endothelial cells. These inclusions were evident in all SIV-infected macaques (magnification, ×3,800).
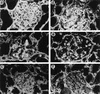
FIG. 3
Increased type IV and I collagen deposition in macaques infected with SIVmacR71/17E. Acetone-fixed frozen sections from macaques AQ47 (group 1 macaque with severe glomerulosclerosis), AQ43 (group 2 macaque with minimal renal pathology), and Y (group 3, uninfected) were stained for collagen type IV (a to c) and type I (d to f) as described in Materials and Methods. (a) Immunofluorescence staining for collagen IV of a sclerotic glomerulus from AQ47 showing a relatively homogeneous, dense staining of collagen IV throughout the glomerulus; (b) immunofluorescence staining for collagen IV of a glomerulus from AQ43 showing a foci of dense staining of collagen IV within the glomerulus; (c) immunofluorescence staining for collagen IV of a normal glomerulus from uninfected macaque Y showing a relatively light homogeneous staining for collagen IV; (d) immunofluorescence staining for collagen I of a sclerotic glomerulus from AQ47 showing a relatively homogeneous, dense staining of collagen I throughout the glomerulus; (e) immunofluorescence staining for collagen IV of a glomerulus from AQ43 showing a foci of dense staining of collagen I within the glomerulus; (f) immunofluorescence staining for collagen I of a normal glomerulus from uninfected macaque Y to demonstrate the distribution of collagens from data in Tables 3 and 4. It can be seen that there was relatively little glomerular staining of collagen I compared to that evident in infected macaques. (Magnification of all panels, ×100.)
FIG. 4
Deposition of immunoglobulin in the glomeruli of macaques infected with SIVmacR71/17E. Acetone-fixed frozen sections from representative macaques were stained for the presence of IgG, IgM, or IgA as described in Materials and Methods. (a) Micrograph showing that IgG is present largely within the mesangial region of this sclerotic glomerulus from macaque AQ20. (b) Micrograph showing that IgM is localized largely to the mesangial region of this sclerotic glomerulus from AQ20. (c) IgA is present within this glomerulus from AQ20. (d) Micrograph showing that there is no IgG (nor IgM or IgA [data not shown]) in the glomeruli from uninfected macaque Y. (Magnification of all panels, ×100.)
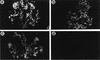
FIG. 5
Glomerular SIVmacp27 antigen in macaques infected with SIVmacR71/17E. Acetone-fixed frozen sections of kidney were prepared and stained for the presence of p27 antigen as described in Materials and Methods. (a) Low-magnification immunofluorescence micrograph of AQ47 showing that the glomeruli are the only structures stained (magnification, ×25). (b) Higher magnification of the same section showing that p27 staining is relatively uniform throughout the glomerulus. The oval dark regions appear to be the nuclei of glomerular cells. This pattern of staining is in sharp contract to the multifocal nature of the staining for macrophages (compare to the CD68+ cells in Fig. 6b). Therefore, the p27 staining appears to be in more cells than can be explained on the basis of resident glomerular macrophages (magnification, ×100). (c) The glomerulus shown in panel b was stained for IgG and shows the diffuse but localized deposits of IgG. Since the patterns of distribution of p27 and IgG do not appear to parallel each other, it is unlikely that the p27 staining can be explained solely on the basis of glomerular deposition of IgG-p27 immune complexes (magnification, ×100).
FIG. 5
Glomerular SIVmacp27 antigen in macaques infected with SIVmacR71/17E. Acetone-fixed frozen sections of kidney were prepared and stained for the presence of p27 antigen as described in Materials and Methods. (a) Low-magnification immunofluorescence micrograph of AQ47 showing that the glomeruli are the only structures stained (magnification, ×25). (b) Higher magnification of the same section showing that p27 staining is relatively uniform throughout the glomerulus. The oval dark regions appear to be the nuclei of glomerular cells. This pattern of staining is in sharp contract to the multifocal nature of the staining for macrophages (compare to the CD68+ cells in Fig. 6b). Therefore, the p27 staining appears to be in more cells than can be explained on the basis of resident glomerular macrophages (magnification, ×100). (c) The glomerulus shown in panel b was stained for IgG and shows the diffuse but localized deposits of IgG. Since the patterns of distribution of p27 and IgG do not appear to parallel each other, it is unlikely that the p27 staining can be explained solely on the basis of glomerular deposition of IgG-p27 immune complexes (magnification, ×100).
FIG. 6
Macrophage (CD68+ cells) are present in the kidney of macaques infected with SIVmacR71/17E. Acetone-fixed frozen sections from the kidneys of macaques AQ20 (with severe glomerulosclerosis) and AQ43 (with minimal renal pathology) were stained for the presence of CD68+ cells as described in Materials and Methods. (a) Low-magnification micrograph of kidney tissue from AQ20 stained for CD68+ cells (monocytes/macrophages) in which a few glomeruli (arrowheads) are evident. There are a number of CD68+ cells (black foci) scattered throughout the parenchyma (magnification, ×25). (b) Higher magnification of a glomerulus from AQ20 showing several CD68+ cells (magnification, ×100). (c) Low-magnification micrograph of kidney from AQ43 stained for CD68+ cells (monocytes/macrophages). A few glomeruli (arrowheads) are evident, as are a number of CD68+ cells (black foci) scattered throughout the parenchyma (magnification, ×25). (d) Higher magnification of a glomerulus from AQ43 with only a few CD68+ cells (magnification, ×100).
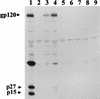
FIG. 7
Immunoprecipitation of SIVmac proteins with serum samples taken from macaques at necropsy. SIVmacR71/17E (104 TCID50) was used to inoculate 2 × 106 CEM174 cells. At 4 days postinoculation, cells were starved for methionine and cysteine and then radiolabeled with 1,000 μCi of [35S]methionine and cysteine for 18 h. The culture supernatant was retained, and SIV proteins were immunoprecipitated with 10 μl of each serum sample and protein A-Sepharose as described in the text. Immunoprecipitates were washed three times in radioimmunoprecipitation assay buffer, samples were denatured by boiling in SDS-PAGE sample reducing buffer, and proteins were separated by SDS-PAGE (10% gel). Proteins were visualized by standard autoradiographic techniques. Lanes: 1, SIV proteins immunoprecipitated from a macaque infected with SIVmac239; 2, SIV proteins immunoprecipitated with a serum from an uninfected macaque; 3, SIV proteins immunoprecipitated with serum from macaque AQ70; 4, SIV proteins immunoprecipitated with serum from macaque AQ69; 5, SIV proteins immunoprecipitated with serum from macaque AQ47; 6, SIV proteins immunoprecipitated with serum from macaque AQ43; 7, SIV proteins immunoprecipitated with serum from macaque AQ38; 8, SIV proteins immunoprecipitated with serum from macaque AQ20; 9, SIV proteins immunoprecipitated with serum from macaque AQ12.
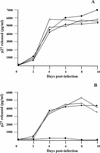
FIG. 8
Interviral recombinants constructed with the gp120 regions isolated from the glomerular fractions of macaques AQ20 and AQ47 are M-tropic. Interviral recombinants were constructed as described in Materials and Methods and used to inoculate either CEMx174 or rhesus macaque macrophage cultures. (A) Growth curves of SIVmac239, SIVmacR71/17E, SIVmacAQ20GLO, and SIVmacAQ47GLO in CEMx174 cultures. CEMx174 cells (106 cells/culture) were inoculated with 1,000 TCID50 of each virus (multiplicity of infection of approximately 0.001) for 24 h, washed three times to remove the virus inoculum, and maintained in the appropriate medium for the course of the infection. Culture medium was harvested at the time points indicated and assayed for the presence of p27 antigen as described in Materials and Methods. •, SIVmac239-inoculated CEMx174 cultures; ○, SIVmacR71/17E-inoculated CEMx174 cultures; ■, SIVmacAQ20GLO-inoculated CEMx174 cultures; □, SIVmacAQ47GLO-inoculated CEMx174 cultures. (B) Growth curves of SIVmac239, SIVmacLG1, SIVmacAQ20GLO, and SIVmacAQ47GLO in macrophage cultures. Rhesus macrophages in 35-mm-diameter dishes were prepared as described earlier (58). All cultures were inoculated, washed, and maintained as described above. Culture medium was harvested at the time points indicated and assayed for the presence of p27 antigen by using antigen capture assays (Coulter Corp.). •, SIVmac239-inoculated rhesus macrophage cultures; ○, SIVmacR71/17E-inoculated rhesus macrophage cultures; ■, SIVmacAQ20GLO-inoculated rhesus macrophage cultures; □, SIVmacAQ47GLO-inoculated rhesus macrophage cultures.
Similar articles
- SIV-associated nephropathy in rhesus macaques infected with lymphocyte-tropic SIVmac239.
Gattone VH 2nd, Tian C, Zhuge W, Sahni M, Narayan O, Stephens EB. Gattone VH 2nd, et al. AIDS Res Hum Retroviruses. 1998 Sep 1;14(13):1163-80. doi: 10.1089/aid.1998.14.1163. AIDS Res Hum Retroviruses. 1998. PMID: 9737588 - Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus.
Clements JE, Montelaro RC, Zink MC, Amedee AM, Miller S, Trichel AM, Jagerski B, Hauer D, Martin LN, Bohm RP, et al. Clements JE, et al. J Virol. 1995 May;69(5):2737-44. doi: 10.1128/JVI.69.5.2737-2744.1995. J Virol. 1995. PMID: 7707496 Free PMC article. - Lymphocyte-tropic simian immunodeficiency virus causes persistent infection in the brains of rhesus monkeys.
Stephens EB, Liu ZQ, Zhu GW, Adany I, Joag SV, Foresman L, Berman NE, Narayan O. Stephens EB, et al. Virology. 1995 Nov 10;213(2):600-14. doi: 10.1006/viro.1995.0032. Virology. 1995. PMID: 7491784 - Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV).
Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, Cheng-Mayer C. Luciw PA, et al. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7490-4. doi: 10.1073/pnas.92.16.7490. Proc Natl Acad Sci U S A. 1995. PMID: 7638218 Free PMC article. - Acquired immunodeficiency syndrome and the blood-brain barrier.
Ivey NS, MacLean AG, Lackner AA. Ivey NS, et al. J Neurovirol. 2009 Apr;15(2):111-22. doi: 10.1080/13550280902769764. J Neurovirol. 2009. PMID: 19306229 Free PMC article. Review.
Cited by
- Controversies in the pathogenesis of HIV-associated renal diseases.
Bruggeman LA, Nelson PJ. Bruggeman LA, et al. Nat Rev Nephrol. 2009 Oct;5(10):574-81. doi: 10.1038/nrneph.2009.139. Nat Rev Nephrol. 2009. PMID: 19776779 Free PMC article. Review. - Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models.
de Mik SM, Hoogduijn MJ, de Bruin RW, Dor FJ. de Mik SM, et al. BMC Nephrol. 2013 Apr 1;14:74. doi: 10.1186/1471-2369-14-74. BMC Nephrol. 2013. PMID: 23547922 Free PMC article. Review. - The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses.
Bannert N, Schenten D, Craig S, Sodroski J. Bannert N, et al. J Virol. 2000 Dec;74(23):10984-93. doi: 10.1128/jvi.74.23.10984-10993.2000. J Virol. 2000. PMID: 11069993 Free PMC article. - HIV-1 Infection of T Lymphocytes and Macrophages Affects Their Migration via Nef.
Vérollet C, Le Cabec V, Maridonneau-Parini I. Vérollet C, et al. Front Immunol. 2015 Oct 6;6:514. doi: 10.3389/fimmu.2015.00514. eCollection 2015. Front Immunol. 2015. PMID: 26500651 Free PMC article. Review. - HIV-1-induced AIDS in monkeys.
Hatziioannou T, Del Prete GQ, Keele BF, Estes JD, McNatt MW, Bitzegeio J, Raymond A, Rodriguez A, Schmidt F, Mac Trubey C, Smedley J, Piatak M Jr, KewalRamani VN, Lifson JD, Bieniasz PD. Hatziioannou T, et al. Science. 2014 Jun 20;344(6190):1401-5. doi: 10.1126/science.1250761. Science. 2014. PMID: 24948736 Free PMC article.
References
- Bodi I, Abraham A A, Kimmel P L. Macrophages in human immunodeficiency virus-associated kidney diseases. Am J Kidney Dis. 1994;24:762–767. - PubMed
- Bottomley S P, Beckingham J A, Murphy J P, Atkinson M, Hinton R J, Gore M G. Cloning, expression, and purification of Ppl-1, a kappa-chain binding protein, based upon protein L from Peptostreptococcus magnus. Bioseparation. 1995;5:359–367. - PubMed
- Bourgoignie J J. Renal complications of human immunodeficiency virus type 1. Kidney Int. 1990;37:1571–1584. - PubMed
- Bourgoignie J J, Pardo V. The nephropathology in human immunodeficiency virus (HIV-1) infection. Kidney Int. 1991;40:19–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous