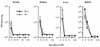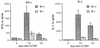Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice - PubMed (original) (raw)
Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice
D Homann et al. J Virol. 1998 Nov.
Abstract
Adoptive transfer of virus-specific memory lymphocytes can be used to identify factors and mechanisms involved in the clearance of persistent virus infections. To analyze the role of B cells in clearing persistent infection with lymphocytic choriomeningitis virus (LCMV), we used B-cell-deficient muMT/muMT (B-/-) mice. B-/- mice controlled an acute LCMV infection with the same kinetics and efficiency as B-cell-competent (B+/+) mice via virus-specific, major histocompatibility complex (MHC) class I-restricted CD8(+) cytotoxic T lymphocytes (CTL). CTL from B-/- and B+/+ mice were equivalent in affinity to known LCMV CTL epitopes and had similar CTL precursor frequencies (pCTL). Adoptive transfer of memory cells from B+/+ mice led to virus clearance from persistently infected B+/+ recipients even after in vitro depletion of B cells, indicating that B cells or immunoglobulins are not required in the transfer population. In contrast, transfer of memory splenocytes from B-/- mice failed to clear virus. Control of virus was restored neither by transferring higher numbers of pCTL nor by supplementing B-/- memory splenocytes with LCMV-immune B cells or immune sera. Instead, B-/- mice were found to have a profound CD4 helper defect. Furthermore, compared to cultured splenocytes from B+/+ mice, those from B-/- mice secreted less gamma interferon (IFN-gamma) and interleukin 2, with differences most pronounced for CD8 T cells. While emphasizing the importance of CD4 T-cell help and IFN-gamma in the control of persistent infections, the CD4 T-helper and CD8 T-cell defects in B-/- mice suggest that B cells contribute to the induction of competent T effector cells.
Figures
FIG. 1
B−/− mice control acute infection with LCMV. Mice (6 to 8 weeks old) were inoculated i.p. with 105 PFU of LCMV ARM, and virus titers were determined 3, 7, 15, 60, 120, and 210 days later in a standard plaque assay on Vero cells. The detection level was 200 PFU. Data are averages ± 1 SE for three B+/+ and three B−/− mice.
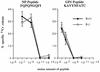
FIG. 2
B−/− and B+/+ CTL display similar CTL affinities to dominant LCMV epitopes. CTL assays were performed 7 days after i.p. infection of mice with 105 PFU of LCMV ARM as described in Materials and Methods. Briefly, splenic lymphocyte effectors were used at an E:T ratio of 50:1; targets were MC57 H-2b mouse fibroblasts coated immediately before addition of effectors with LCMV NP peptide FQPQNGQFI (aa 396 to 404) or GP1 peptide KAVYNFATC (aa 33 to 41) at dilutions ranging from 10−6 to 10−13. Results were obtained in a 5- to 6-h 51Cr release assay; percent killing of uncoated targets was subtracted from specific killing. Values are means ± 1 SE for three to four mice per each group.
FIG. 3
B−/− memory splenocytes fail to clear virus from persistently infected hosts. (A) B+/+ and B−/− mice show similar pCTL frequencies 60 days after acute infection with LCMV; three individual mice of each group, as well as pooled splenocytes for adoptive transfers, which yielded similar results (data not shown), were analyzed. (B) Adoptive transfer of B−/− memory splenocytes fails to clear virus from sera of persistently infected B+/+ recipients. Transfers of as many as 108 B−/− splenocyte populations were conducted to ensure that a sufficient number of CTL precursors was contained in the transfer population. Control mice received 3 × 107 B+/+ memory splenocytes. Experimental groups consisted of two to four mice. (C) Transfer of B−/− or B+/+ memory splenocytes into persistently infected B−/− recipients failed to clear virus from recipients. Experimental groups consisted of three to nine mice. Data are means ± 1 SE.
FIG. 4
Virus persists after transfer of B−/− memory splenocytes combined with LCMV-primed B cells or LCMV-immune serum but not B-cell-depleted B+/+ memory splenocytes. (A) B−/− memory splenocytes reconstituted with either LCMV-primed B cells or LCMV-immune serum did not clear virus. LCMV-immune sera were obtained from B+/+ splenocyte donors 60 days after acute infection, and 0.2 ml was given i.p. on days 0, 5, and 11 after adoptive transfers. LCMV-specific sera contained an ELISA anti-LCMV IgG titer of 1:43,740 but little to no neutralizing antibody (data not shown). Experimental groups consisted of four mice. Controls included persistently infected recipients receiving LCMV-immune serum only, as well as B+/+ populations. (B) Adoptive transfer of 3 × 107 B-cell-depleted B+/+ memory splenocytes led to long-term virus clearance from serum in persistently infected B+/+ recipients. Three to four mice were analyzed at each time point. Data are means ± 1 SE.
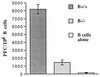
FIG. 5
In vitro CD4 helper activity from B−/− and B+/+ mice. CD4 T cells from B−/− mice exhibit decreased in vitro helper activity. Ten days after immunization with 100 μg of HGG in CFA, CD4 T cells were cultured with TNP-KLH-primed B cells and 10 ng of TNP-HGG. Anti-TNP PFC with TNP-SRBC targets were measured on day 7 of culture.
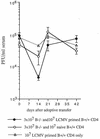
FIG. 6
Reconstitution of B−/− memory splenocytes with LCMV-primed CD4 cells temporarily decreased virus titers by 1.5 log units. Reconstitution with naive B+/+ CD4 cells showed a less-pronounced effect. Four to five mice were analyzed per group; one control recipient received only LCMV-primed CD4 cells.
FIG. 7
Differential cytokine production by B+/+ and B−/− splenocytes after antigen-specific stimulation in vitro. Supernatant of 6 × 106 splenocytes cultured for 2 days with LCMV-infected and irradiated macrophages was analyzed by a sandwich ELISA. Splenocytes from uninfected B+/+ and B−/− mice did not produce detectable levels of IFN-γ or IL-2. B+/+ splenocytes produced significantly more IFN-γ at all time points after infection with LCMV (P < 0.05). At 30 and 60 days after acute LCMV infection, B+/+ splenocytes also produced more IL-2 (P < 0.01).
Similar articles
- A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers.
Planz O, Ehl S, Furrer E, Horvath E, Bründler MA, Hengartner H, Zinkernagel RM. Planz O, et al. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6874-9. doi: 10.1073/pnas.94.13.6874. Proc Natl Acad Sci U S A. 1997. PMID: 9192659 Free PMC article. - CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection.
Matloubian M, Concepcion RJ, Ahmed R. Matloubian M, et al. J Virol. 1994 Dec;68(12):8056-63. doi: 10.1128/JVI.68.12.8056-8063.1994. J Virol. 1994. PMID: 7966595 Free PMC article. - CD8 T cell memory in B cell-deficient mice.
Asano MS, Ahmed R. Asano MS, et al. J Exp Med. 1996 May 1;183(5):2165-74. doi: 10.1084/jem.183.5.2165. J Exp Med. 1996. PMID: 8642326 Free PMC article. - Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.
Studstill CJ, Hahm B. Studstill CJ, et al. Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review. - Lymphocytic choriomeningitis infection of the central nervous system.
Kang SS, McGavern DB. Kang SS, et al. Front Biosci. 2008 May 1;13:4529-43. doi: 10.2741/3021. Front Biosci. 2008. PMID: 18508527 Free PMC article. Review.
Cited by
- Role of B cells and the aging brain in stroke recovery and treatment.
Engler-Chiurazzi EB, Monaghan KL, Wan ECK, Ren X. Engler-Chiurazzi EB, et al. Geroscience. 2020 Oct;42(5):1199-1216. doi: 10.1007/s11357-020-00242-9. Epub 2020 Aug 7. Geroscience. 2020. PMID: 32767220 Free PMC article. Review. - Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses.
McClellan KB, Gangappa S, Speck SH, Virgin HW 4th. McClellan KB, et al. PLoS Pathog. 2006 Jun;2(6):e58. doi: 10.1371/journal.ppat.0020058. Epub 2006 Jun 23. PLoS Pathog. 2006. PMID: 16789842 Free PMC article. - CD4+ T cells derived from B cell-deficient mice inhibit the establishment of peripheral B cell pools.
Baumgarth N, Jager GC, Herman OC, Herzenberg LA. Baumgarth N, et al. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4766-71. doi: 10.1073/pnas.97.9.4766. Proc Natl Acad Sci U S A. 2000. PMID: 10781082 Free PMC article. - Induction and function of virus-specific CD4+ T cell responses.
Whitmire JK. Whitmire JK. Virology. 2011 Mar 15;411(2):216-28. doi: 10.1016/j.virol.2010.12.015. Epub 2011 Jan 14. Virology. 2011. PMID: 21236461 Free PMC article. Review.
References
- Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-11576/AI/NIAID NIH HHS/United States
- T32 AG000080/AG/NIA NIH HHS/United States
- R29 DK051091/DK/NIDDK NIH HHS/United States
- AI-09484/AI/NIAID NIH HHS/United States
- R01 AI009484/AI/NIAID NIH HHS/United States
- R-29 DK 51091/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials