Activity and nature of p21(WAF1) complexes during the cell cycle - PubMed (original) (raw)
Activity and nature of p21(WAF1) complexes during the cell cycle
K Cai et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Elevated levels of the p21(WAF1) (p21) cyclin-dependent kinase inhibitor induce growth arrest. We have characterized a panel of monoclonal antibodies against human p21 in an effort to understand the dynamic regulatory interactions between this and other cellular proteins during the cell cycle. The use of these reagents has allowed us to address several important, yet unresolved, issues concerning the biological activity of p21, including the potential kinase activity of complexes that associate with this cyclin-dependent kinase inhibitor. We have found that the kinase activity of cyclin A/Cdk2 associated with p21 is significantly lower than that of cyclin A/Cdk2 free of p21, suggesting that p21 abolishes its activity in vivo, and the use of multiple antibodies has enabled us to begin the study of the molecular architecture of p21 complexes in vivo. In addition, we found that human fibroblasts released from a quiescent state display abundant amounts of p21 devoid of associated proteins ("free" p21), the levels of which decrease as cells approach S phase. Cyclin A levels increase as the amount of monomeric p21 decreases, resulting in an excess of cyclin A/Cdk2 complexes that are not bound to, or inactivated by, p21. Our data strengthen the notion that the G1-to-S phase transition in human fibroblasts occurs when the concentration of cyclin A/Cdk2 surpasses that of p21.
Figures
Figure 1
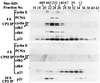
Characterization of anti-p21 antibodies. (A) Schematic representation of functional domains of p21 and the epitopes recognized by anti-p21 antibodies. Epitopes were mapped by using a peptide array as described. On the basis of this analysis, we mapped the antibody recognition to the following epitopes: residues 5–13 (CP2), 19–25 (CP36), 35–43 (CP50), 69–78 (CP55), 96–104 (CP59), and 95–102 (CP68). One antibody, CP50, failed to immunoprecipitate endogenous p21 and was not characterized further. (B) Asynchronous WI38 cell extracts (300 μg) were immunoprecipitated with different anti-p21 antibodies as indicated. The first lane (IN) represents 10% of input protein loaded directly. The last lane shows a control using anti-flu hemagglutinin (HA) mAb, 12CA5. For comparison, recombinant p21 (rp21; 3 ng) from bacteria was immunoprecipitated. (C) Immunoprecipitations of extracts of WI38 cells metabolically labeled with [35S]methionine using the indicated antibodies were resolved by SDS/PAGE and visualized by fluorography. The identity of associated proteins was assigned by parallel immunoprecipitations with antibodies against each of the indicated proteins. Asterisks indicate unidentified proteins. (D) The epitopes recognized by CP36 and CP55 are buried in cellular p21-containing complexes. Asynchronous WI38 cell whole extracts (300 μg) were treated without urea (native or N), or with 0.4 M (N′) or 8 M (refolding or R) urea as described. Samples were then diluted 20-fold with buffer (N and R) or 0.4 M urea (N′) and immunoprecipitated and Western blotted. HA is a control using antibody 12CA5.
Figure 2
The p21/cyclin A/Cdk2 ternary complex is significantly less active than the cyclin A/Cdk2 complex in vivo. Whole cell extracts from exponentially growing WI38 cells were split into two equivalent samples. One half was left untreated, whereas the other was treated with three rounds of CP2-conjugated beads to deplete p21-containing complexes. Immunoprecipitations were performed on both depleted (+) and nondepleted (−) samples using different antibodies as indicated. The immunoprecipitated samples were split again and tested by Western blotting using anti-p21, anti-cyclin A, and anti-Cdk2 antibodies and in histone H1 kinase assays.
Figure 3
Excess free p21 exists exclusively during early G1 phase. (A) WI38 cells were synchronized by serum deprivation, and the cell cycle profile determined by FACS analysis is shown. (B) Whole cell extracts derived from WI38 cells were analyzed directly or by sequential immunoprecipitation according to the scheme shown. (C) Extract (20 μg) from each stage was loaded directly and detected by Western blotting to reveal the total amounts of cyclin A, Cdk2, and p21. (D) Protein (300 μg) was immunoprecipitated by using CP55 to detect free p21 at each cell cycle time point (the first round of three consecutive depletions is shown). (E) Supernatants after three rounds of depletion of free p21 by CP55 were subjected to CP2 immunoprecipitation to detect cyclin A/Cdk2/p21 ternary complexes during the cell cycle. Histone H1 kinase activity was measured in parallel. (F) Supernatants after the immunoprecipitation in E were subjected to immunoprecipitation using anti-cyclin A antibody-conjugated beads to detect the remaining cyclin A/Cdk2 complex. Relative Western blot exposure times are the same for each part of the figure. Histone H1 kinase activity was measured in parallel.
Figure 4
Free p21 in WI38 cells 4 hr after serum restimulation revealed by sizing column chromatography. An S-300 column was used to separate p21 and its complexes as described. The elution of calibration standards is indicated at the top. Protein (500 μg) derived from extracts of cells 4 or 24 hr after serum stimulation were loaded onto the column. Fractions were collected, halved, and immunoprecipitated with either CP2 or CP55 as labeled. The samples were analyzed by Western blotting to reveal p21 and its complexes.
Similar articles
- Caco-2 intestinal cell differentiation is associated with G1 arrest and suppression of CDK2 and CDK4.
Ding QM, Ko TC, Evers BM. Ding QM, et al. Am J Physiol. 1998 Nov;275(5):C1193-200. doi: 10.1152/ajpcell.1998.275.5.C1193. Am J Physiol. 1998. PMID: 9814966 - Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells.
Orend G, Hunter T, Ruoslahti E. Orend G, et al. Oncogene. 1998 May;16(20):2575-83. doi: 10.1038/sj.onc.1201791. Oncogene. 1998. PMID: 9632134 - [E1A oncogene effect on the ability of p21(Waf1) to regulate G1/S arrest in E1A-expressing transformants following irradiation].
Romanov VS, Brichkina AI, Pospelov VA, Pospelova TV. Romanov VS, et al. Tsitologiia. 2005;47(12):1063-70. Tsitologiia. 2005. PMID: 16706194 Russian.
Cited by
- Human cyclin A is required for mitosis until mid prophase.
Furuno N, den Elzen N, Pines J. Furuno N, et al. J Cell Biol. 1999 Oct 18;147(2):295-306. doi: 10.1083/jcb.147.2.295. J Cell Biol. 1999. PMID: 10525536 Free PMC article. - DIXDC1 targets p21 and cyclin D1 via PI3K pathway activation to promote colon cancer cell proliferation.
Wang L, Cao XX, Chen Q, Zhu TF, Zhu HG, Zheng L. Wang L, et al. Cancer Sci. 2009 Oct;100(10):1801-8. doi: 10.1111/j.1349-7006.2009.01246.x. Epub 2009 Jun 17. Cancer Sci. 2009. PMID: 19572978 Free PMC article. - PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule?
Soria G, Gottifredi V. Soria G, et al. DNA Repair (Amst). 2010 Apr 4;9(4):358-64. doi: 10.1016/j.dnarep.2009.12.003. Epub 2010 Jan 8. DNA Repair (Amst). 2010. PMID: 20060369 Free PMC article. Review. - Serum starvation induced cell cycle synchronization facilitates human somatic cells reprogramming.
Chen M, Huang J, Yang X, Liu B, Zhang W, Huang L, Deng F, Ma J, Bai Y, Lu R, Huang B, Gao Q, Zhuo Y, Ge J. Chen M, et al. PLoS One. 2012;7(4):e28203. doi: 10.1371/journal.pone.0028203. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529890 Free PMC article. - Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases.
Blomberg I, Hoffmann I. Blomberg I, et al. Mol Cell Biol. 1999 Sep;19(9):6183-94. doi: 10.1128/MCB.19.9.6183. Mol Cell Biol. 1999. PMID: 10454565 Free PMC article.
References
- Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. - PubMed
- Gu Y, Turck C W, Morgan D O. Nature (London) 1993;366:707–710. - PubMed
- Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. - PubMed
- Goubin F, Ducommun B. Oncogene. 1995;10:2281–2287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources