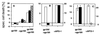CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells - PubMed (original) (raw)
CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells
C Berndt et al. Proc Natl Acad Sci U S A. 1998.
Abstract
AIDS is characterized by a progressive decrease of CD4(+) helper T lymphocytes. Destruction of these cells may involve programmed cell death, apoptosis. It has previously been reported that apoptosis can be induced even in noninfected cells by HIV-1 gp120 and anti-gp120 antibodies. HIV-1 gp120 binds to T cells via CD4 and the chemokine coreceptor CXCR4 (fusin/LESTR). Therefore, we investigated whether CD4 and CXCR4 mediate gp120-induced apoptosis. We used human peripheral blood lymphocytes, malignant T cells, and CD4/CXCR4 transfectants, and found cell death induced by both cell surface receptors, CD4 and CXCR4. The induced cell death was rapid, independent of known caspases, and lacking oligonucleosomal DNA fragmentation. In addition, the death signals were not propagated via p56(lck) and Gialpha. However, the cells showed chromatin condensation, morphological shrinkage, membrane inversion, and reduced mitochondrial transmembrane potential indicative of apoptosis. Significantly, apoptosis was exclusively observed in CD4(+) but not in CD8(+) T cells, and apoptosis triggered via CXCR4 was inhibited by stromal cell-derived factor-1, the natural CXCR4 ligand. Thus, this mechanism of apoptosis might contribute to T cell depletion in AIDS and might have major implications for therapeutic intervention.
Figures
Figure 1
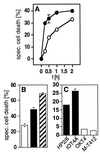
CD4 and CXCR4 mediate a rapid type of cell death in human cells. (A) Kinetics of anti-CD4- (•) and anti-CXCR4-induced (○) cell death in HPB-ALL cells. Cells were preincubated with the anti-CD4 mAb HP2/6 or the anti-CXCR4 mAb 12G5 for 15 min at 37°C, then receptors were crosslinked by coated sheep-anti-mouse Abs (100 μg/ml). Cell death was determined by FSC/SSC analysis at the indicated time points. One representative experiment of three performed in triplicates is shown. (B) Induction of cell death in HPB-ALL cells by mAbs to CXCR4 (□), to CD4 (■), or to both receptors (▨), respectively. Induction of cell death with both mAbs was additive. Cell death was induced as described in Materials and Methods and was determined 2 h after mAb exposure by FSC/SSC analysis. One experiment representative of two, done in triplicates, is shown. (C) CD4-mediated cell death in HPB-ALL cells is triggered only by mAbs that interfere with gp120 binding, i.e. the anti-CD4 mAbs HP2/6 or IOT4A (■). The anti-CD4 mAbs OKT4 and M-T412 (□) that do not interfere with gp120 binding did not induce cell death. Cell death was determined after 2 h by FSC/SSC analysis. One of two experiments with similar results, done in triplicates, is shown. Panels are expressed as % specific cell death. Background was between 6.63% and 9.49%.
Figure 2
(A) Cell death induced by anti-CD4 or anti-CXCR4 mAbs is not inhibited by the caspase inhibitor zVAD-fmk. HPB-ALL cells or anti-CD95-sensitive SKW6.4 cells were incubated in the absence (□) or presence (■) of 100 μM zVAD-fmk for 30 min at 37°C. Cell death was determined 2 h after triggering with anti-CD4 or anti-CXCR4 mAbs or 24 h after triggering with anti-APO-1 mAbs by FSC/SSC analysis. (B) DNA fragmentation is not observed during CD4- and CXCR4-mediated apoptosis. HPB-ALL cells (5 × 105) were incubated for 2 h with anti-CD4 or anti-CXCR4 mAbs or for 96 h with 10 μg/ml puromycin. Formation of subdiploid nuclei was determined after overnight incubation by propidium iodide staining. (C) Loss of asymmetry in plasma membrane phospholipids precedes loss of cell viability in anti-CD4- and anti-CXCR4-induced apoptosis. At the indicated time points after induction of cell death, HPB-ALL cells were harvested and stained with propidium iodide (○) or the lipophilic dye MC540 (•). The values are the mean ± SD of triplicates of one of two similar experiments. (D) Reduction of ΔΨm occurs during CD4- (○) and CXCR4-mediated (•) cell death. ΔΨm was determined in comparison to anti-mouse-Ig-treated controls at the indicated time points after apoptosis induction in HPB-ALL cells. Results are representative of three independent experiments. (E) Apoptotic HPB-ALL cells show changes in FSC/SSC typical of apoptosis. The values represent the percentage of apoptotic cells with reduced FSC and increased SSC (gated cells). Cell death was induced as described and determined after 2 h. Experiments were done in triplicates more than five times. One representative example of each group is shown. (F) Anti-CD4- and anti-CXCR4-treated HPB-ALL cells show condensed cytoplasm and condensed chromatin. Representative electron micrographs of HPB-ALL cells (×6,000) treated for 25 min with secondary Abs alone, anti-CXCR4 or anti-CD4 mAbs are shown. Condensation was observed in 0 of 127 (0%) control cells, 21 of 84 (25%) anti-CXCR4- and 46 of 120 (38%) anti-CD4-treated cells. The experiment was repeated twice with similar results.
Figure 3
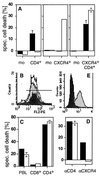
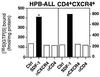
CD4 and CXCR4 induce cell death independently of each other. (A) Anti-CD4-induced cell death (■) was observed in all CD4+ transfectants of the human B lymphoma cells tested. All CXCR4+ transfectants obtained were sensitive to anti-CXCR4-induced cell death (□). Double transfectants were sensitive to cell death induced by both mAbs. One representative example of every group of transfectants is shown. mean fluorescence intensity of CD4/CXCR4 of the clones used was: mock (3.85/2.57), CD4+ (47.12/2.56), CXCR4+ (4.03/65.7), and CD4+CXCR4+ (76.72/102.44). The killing assay was performed five times in triplicates as described in Materials and Methods. (B) CD8+ T cells (dark gray peak) express more CXCR4 than CD4+ T cells (light gray peak) (PBL = white peak). (C) Only CD4+ T cells are killed by anti-CD4 (■) and anti-CXCR4 (□) mAbs. Human PBL were sorted by negative selection with magnetic beads and cell death was induced. The experiment shown was done in triplicates and is representative of five independent experiments. (D) Down-regulation of CXCR4 by SDF-1 prevents anti-CXCR4- but not anti-CD4-induced cell death. HPB-ALL cells were preincubated in the absence (■) or presence (□) of 250 nM SDF-1 for 30 min at 37°C. The assay was performed in triplicates. One of five similar experiments is shown. (E) Down-regulation of CXCR4 was controlled by surface staining. White peak, control antibody; light gray peak, CXCR4 staining; dark gray peak, CXCR4 staining after preincubation with SDF-1 (24). Cell death in A, C, and D was determined by FSC/SSC analysis after 2 h.
Figure 4
CD4- and CXCR4-mediated apoptosis does not involve Giα activation. Binding of GTP[γ-35S] to membranes of HPB-ALL cells and CD4+CXCR4+ transfectants was assayed in the absence (Ctrl) or presence of SDF-1 (200 nM), anti-CXCR4 (10 μg/ml), or anti-CD4 mAbs (10 μg/ml). The experiment shown was done in triplicate and is a representative of three experiments done.
Figure 5
The features of gp120/anti-gp120-induced apoptosis in human PBL are identical to anti-CD4- or anti-CXCR4-induced apoptosis. (A) gp120/anti-gp120-induced cell death in human PBL induced changes in FSC/SSC typical of apoptosis (□). Furthermore, loss of asymmetry in plasma membrane phospholipids as determined by MC540 staining (▨) preceded loss of cell viability as determined by propidium iodide staining (■). After preincubation with 100 ng/ml gp120 (HIV-1 IIIB, Neosystem, Strasbourg, France) for 60 min at 4°C, PBL (5 × 105 cells per sample) were incubated with diluted rabbit anti-gp120 serum (1/500). Cell death was determined after 30 min. The experiment was done in triplicate and is representative of three. (B) gp120/anti-gp120-induced cell death in human PBL does not result in formation of subdiploid nuclei. Cell death was induced in activated CD95-sensitive PBL as described in A. Anti-APO-1 (10 μg/ml) was used as a positive control. Cell death of an aliquot of cells was measured by FSC/SSC analysis (104 cells counted) after 2 h (gp120/anti-gp120) or 24 h (anti-APO-1) (□). The remaining cells were incubated overnight in propidium iodide-lysis buffer and formation of subdiploid nuclei (■) was determined in a FACScan cytometer (104 cells counted) (20). The experiment shown is a representative of two experiments done. (C) gp120/anti-gp120-induced apoptosis did not involve the known caspases. Before induction of cell death as described in B human PBL were pretreated in the absence (□) or presence (■) of the caspase inhibitor zVAD-fmk (20 μM) for 30 min at 37°C. Cell death was determined by FSC/SSC analysis 2 h (gp120/αgp120) or 24 h (αAPO-1) after induction. One experiment representative of three, done in triplicates, is shown.
Similar articles
- gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors.
Catani MV, Corasaniti MT, Navarra M, Nisticò G, Finazzi-Agrò A, Melino G. Catani MV, et al. J Neurochem. 2000 Jun;74(6):2373-9. doi: 10.1046/j.1471-4159.2000.0742373.x. J Neurochem. 2000. PMID: 10820198 - Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120).
Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Biard-Piechaczyk M, et al. Virology. 2000 Mar 15;268(2):329-44. doi: 10.1006/viro.1999.0151. Virology. 2000. PMID: 10704341 - Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis?
Herbeuval JP, Shearer GM. Herbeuval JP, et al. AIDS Rev. 2006 Jan-Mar;8(1):3-8. AIDS Rev. 2006. PMID: 16736946 Review. - Autophagy and CD4+ T lymphocyte destruction by HIV-1.
Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Espert L, et al. Autophagy. 2007 Jan-Feb;3(1):32-4. doi: 10.4161/auto.3275. Epub 2007 Jan 14. Autophagy. 2007. PMID: 17012832 Review.
Cited by
- Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases.
Risso V, Lafont E, Le Gallo M. Risso V, et al. Cell Death Dis. 2022 Mar 17;13(3):248. doi: 10.1038/s41419-022-04688-x. Cell Death Dis. 2022. PMID: 35301281 Free PMC article. Review. - Computing of Low Shear Stress-Driven Endothelial Gene Network Involved in Early Stages of Atherosclerotic Process.
Vozzi F, Campolo J, Cozzi L, Politano G, Di Carlo S, Rial M, Domenici C, Parodi O. Vozzi F, et al. Biomed Res Int. 2018 Sep 25;2018:5359830. doi: 10.1155/2018/5359830. eCollection 2018. Biomed Res Int. 2018. PMID: 30356351 Free PMC article. - The anti-caspase inhibitor Q-VD-OPH prevents AIDS disease progression in SIV-infected rhesus macaques.
Laforge M, Silvestre R, Rodrigues V, Garibal J, Campillo-Gimenez L, Mouhamad S, Monceaux V, Cumont MC, Rabezanahary H, Pruvost A, Cordeiro-da-Silva A, Hurtrel B, Silvestri G, Senik A, Estaquier J. Laforge M, et al. J Clin Invest. 2018 Apr 2;128(4):1627-1640. doi: 10.1172/JCI95127. Epub 2018 Mar 19. J Clin Invest. 2018. PMID: 29553486 Free PMC article. - Flow cytometry analysis of cell population dynamics and cell cycle during HIV-1 envelope-mediated formation of syncytia in vitro.
Torres-Castro I, Cortés-Rubio CN, Sandoval G, Lamoyi E, Larralde C, Huerta L. Torres-Castro I, et al. In Vitro Cell Dev Biol Anim. 2014;50(5):453-63. doi: 10.1007/s11626-013-9724-z. Epub 2014 Jan 18. In Vitro Cell Dev Biol Anim. 2014. PMID: 24442370 - HIV integrase and the swan song of the CD4 T cells?
Estaquier J, Zaunders J, Laforge M. Estaquier J, et al. Retrovirology. 2013 Dec 9;10:149. doi: 10.1186/1742-4690-10-149. Retrovirology. 2013. PMID: 24321528 Free PMC article.
References
- Fauci A S. Science. 1988;239:617–622. - PubMed
- Heeney J L. Immunol Today. 1995;16:515–520. - PubMed
- Gougeon M L, Lecoeur H, Dulioust A, Enouf M G, Crouvoisier M, Goujard C, Debord T, Montagnier L. J Immunol. 1996;159:3509–3520. - PubMed
- Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Nat Med. 1995;1:129–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous