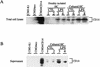Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia - PubMed (original) (raw)
Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia
S Liu et al. Infect Immun. 1998 Nov.
Abstract
Studies were undertaken to examine hepatocyte CD14 expression during endotoxemia. Our results show that lipopolysaccharide (LPS) treatment in vivo caused a marked upregulation in CD14 mRNA and protein levels in rat hepatocytes. Detectable increases in mRNA were seen as early as 1.5 h after LPS treatment; these increases peaked at 20-fold by 3 h and returned to baseline levels by 24 h. In situ hybridization localized the CD14 mRNA expression to hepatocytes both in vitro and in vivo. Increases in hepatic CD14 protein levels were detectable by 3 h and peaked at 12 h. Hepatocytes from LPS-treated animals expressed greater amounts of cell-associated CD14 protein, and more of the soluble CD14 was released by hepatocytes from LPS-treated rats in vitro. The increases in hepatocyte CD14 expression during endotoxemia occurred in parallel to increases of CD14 levels in plasma. To provide molecular identification of the hepatocyte CD14, we cloned the rat liver CD14 cDNA. The longest clone consists of a 1,591-bp insert containing a 1,116-bp open reading frame. The deduced amino acid sequence is 372 amino acids long, has 81.8 and 62.8% homology to the amino acid sequences of mouse and human CD14, respectively, and is identical to the rat macrophage CD14. The expressed CD14 protein from this clone was functional, as indicated by NF-kappaB activation in response to LPS and fluorescein isothiocyanate-LPS binding in CHO cells stably transfected with rat CD14. A nuclear run-on assay showed that CD14 transcription rates were significantly increased in hepatocytes from LPS-treated animals, indicating that the upregulation in CD14 mRNA levels observed in rat hepatocytes after LPS treatment is dependent, in part, on increased transcription. In vitro and in vivo experiments indicated that interleukin-1beta and/or tumor necrosis factor alpha participate in the upregulation of CD14 mRNA levels in hepatocytes. Our data indicate that hepatocytes express CD14 and that hepatocyte CD14 mRNA and protein levels increase rapidly during endotoxemia. Our observations also support the idea that soluble CD14 is an acute-phase protein and that hepatocytes could be a source for soluble CD14 production.
Figures
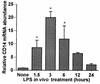
FIG. 1
Time course of hepatocyte CD14 mRNA induction during endotoxemia. Sprague-Dawley rats were injected with LPS (10 mg/kg, i.p.). Hepatocytes were isolated at 1.5, 3, 6, 12, and 24 h from either LPS-treated or control rats. Total RNA was extracted, and Northern blot analysis was performed for CD14 mRNA. Membranes were rehybridized with probe for 18S rRNA. The levels of CD14 mRNA were normalized to 18S rRNA and are presented as the fold increase over the controls. Each column represents the mean ± the SE of triplicate samples at each time point (∗, P < 0.05 versus control).
FIG. 2
Steady-state CD14 mRNA levels in liver tissue after LPS injection. Sprague-Dawley rats were injected with LPS (10 mg/kg, i.p.). Total RNA was extracted from the liver at 1.5, 3, 6, 12, and 24 h after LPS injection, and Northern blot analysis was performed for CD14 mRNA. Membranes were rehybridized with a probe for 18S rRNA. The bar graph shows the levels of CD14 mRNA normalized to 18S rRNA and presented as the fold increase over the controls. The Northern blot is from a representative experiment. Each column represents the mean ± the SE of three independent experiments (∗, P < 0.05 versus control).
FIG. 3
Localization of CD14 mRNA in rat primary hepatocytes and sectioned liver. Hepatocytes grown on coverslips or liver tissue sections were subjected to in situ hybridization with antisense and sense riboprobes for CD14. (A) A differential interference contrast image of individual rat primary hepatocytes (the size bar indicates 20 μm). (B) Dark-field view of the in situ signal (35S). Primary hepatocytes isolated from LPS-treated rats (10 mg/kg, i.p., 24-h treatment) show strong cytoplasmic labeling. In the lower panels, in situ hybridizations performed on sectioned livers from LPS-treated (C) and normal (D) rats are shown. The signal detected with the digoxigenin-labeled riboprobe and colorimetric technique shows labeling in individual hepatocytes around blood vessels, in this case a portal triad. (Positive cells on the original slides were blue and, as shown here, are black.) The number of positive cells decreases with distance from the vasculature. In the control liver (panel D), very few or no positively labeled cells are seen. The sense probe showed little or no labeling (data not shown).
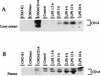
FIG. 4
Western blot analysis of CD14 protein levels in whole-liver extracts (A) and plasma (B) during endotoxemia. Whole livers and plasma samples from LPS-treated rats at 1.5, 3, 6, 12, and 24 h (10 mg/kg, i.p.) or from control rats were prepared. Whole-cell extracts of parental CHO cells and neotransfected CHO cells served as negative controls, and rat CD14-transfected CHO cells were used as a positive control. Proteins (50 μg of whole-liver homogenate or plasma, 1 μg of recombinant proteins per lane) were separated on an SDS–10% polyacrylamide gel and transferred to nitrocellulose membrane. For the detection of CD14 protein, a hamster anti-mouse CD14 monoclonal antibody (G5A10) was incubated at 5 μg/ml with the membranes for 1 h. Immune complexes were detected by enhanced luminol reagent (Dupont, NEN) as described by the manufacturer. The blots are representative of at least three independent experiments. Abbreviations: CHO/rCD14, stably transfected rat CD14 CHO cells; CHO/neo, stably neotransfected CHO cells.
FIG. 5
(A) CD14 protein levels in freshly isolated and cultured hepatocytes. Western blot procedures and abbreviations are as described for Fig. 4. Each group contains duplicate samples from individual animals. Lanes 1 and 2, freshly isolated control hepatocytes; lanes 3 and 4, freshly isolated hepatocytes from LPS-treated rats (10 mg/kg, i.p., 12-h treatment); lanes 5 and 6, control hepatocytes plated for 24 h; lanes 7 and 8, cultured hepatocytes from LPS-treated rats (10 mg/kg, i.p., 12-h treatment) plated for 24 h. (B) CD14 protein levels in hepatocyte culture supernatant. Each group contains duplicate samples from individual animals. Lanes 1 and 2, control hepatocytes plated for 24 h; lanes 3 and 4, cultured hepatocytes from LPS-treated rats (10 mg/kg, i.p., 12-h treatment) plated for 24 h. The data are representative of three independent experiments.
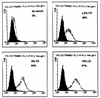
FIG. 6
Serum-dependent binding of FITC-LPS to CHO/rCD14 cells. Cells were stained as described in Materials and Methods and subjected to flow cytometry analysis. The serum concentrations contained in the binding buffer and the percentage of FITC-positive cells are shown. The open histogram represents the FITC-LPS staining. x axis, log fluorescence intensity; y axis, cell number. No FITC-LPS staining was found in CHO-K1 and CHO/neo cells (data not shown). The data are representative of at least three independent experiments.
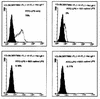
FIG. 7
Competition of FITC-LPS binding to CHO/rCD14 cells by excess of native-form LPS. The binding buffer is PBS with 10% CS and 0.1% sodium azide. The fold excess of native-form LPS added in the binding buffer and the respective percentage of FITC-positive cells are shown. The open histogram represents the FITC-LPS staining. x axis, log fluorescence intensity; y axis, cell number. The data are representative of three independent experiments.
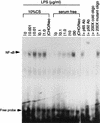
FIG. 8
NF-κB activation by LPS in CHO/rCD14 cells in serum and serum-free conditions. The experiment was carried out as described in Materials and Methods. Cells were treated with different doses of LPS prior to nuclear protein extraction. The doses of LPS for treatment are indicated, except in CHO/neo cells (0.1 μg/ml). Abbreviations: CHO/neo, stably neotransfected CHO cells; p65 Ab, anti-NF-κB p65 subunit antibody; p50 Ab, anti-NF-κB p65 subunit antibody. The data are representative of two independent experiments.
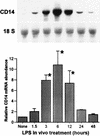
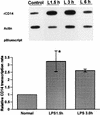
FIG. 9
Nuclear run-on assay in hepatocyte nuclei from LPS-treated rats. Nuclei from control or LPS-injected rats were incubated with [α-32P]UTP to elongate the nascent RNA. The elongated nascent RNA was hybridized to plasmid containing CD14 cDNA immobilized to GeneScreen plus membrane (Dupont, NEN) by a slot-blot apparatus (5 μg/per slot). Equivalent counts per minute of 32P-labeled nuclear RNA probe per milliliter were added to each membrane. Plasmid containing β-actin cDNA and empty vector pBluescript were included as the internal controls. The CD14 transcription rates were quantitated with a PhosphorImager and normalized to that of β-actin. Each column represents the mean ± the SE of duplicate samples (∗, P < 0.05 versus control).
FIG. 10
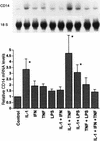
Effects of cytokines on steady-state CD14 mRNA levels in cultured rat hepatocytes. Hepatocyte isolation and Northern blot analysis were carried out as described in Materials and Methods. Rat primary hepatocytes were incubated with single or combined cytokines (500 U/ml) for 12 h. The levels of CD14 mRNA were normalized to 18S rRNA and expressed as the fold increase over the control. Each column represents the mean ± the SE of three independent experiments (∗, P < 0.05 versus control).
FIG. 11
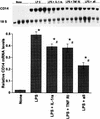
In vivo experiments with IL-1β and TNF-α antagonists. Rats were injected with either saline (vehicle), LPS (10 mg/kg, i.v.), LPS plus IL-1ra (IL-1ra 100 mg/kg, s.c., at time 0 and then every 6 h), LPS plus rsTNF-RI (rsTNF-RI, 1.5 mg/kg, i.v., at time 0), or LPS plus IL-1ra plus sTNF-RI. Total RNA was extracted from the liver 6 h later and Northern blot analysis was performed for CD14 mRNA. The levels of CD14 mRNA were normalized to 18S rRNA and presented as the fold increase over the control. Each column represents the mean ± the SE of four animals in each group (∗, P < 0.05 versus the control; #, P < 0.05 versus the LPS group).
Similar articles
- Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia.
Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Li SW, et al. World J Gastroenterol. 2002 Feb;8(1):124-7. doi: 10.3748/wjg.v8.i1.124. World J Gastroenterol. 2002. PMID: 11833086 Free PMC article. - Hepatocyte toll-like receptor 2 expression in vivo and in vitro: role of cytokines in induction of rat TLR2 gene expression by lipopolysaccharide.
Liu S, Salyapongse AN, Geller DA, Vodovotz Y, Billiar TR. Liu S, et al. Shock. 2000 Sep;14(3):361-5. doi: 10.1097/00024382-200014030-00021. Shock. 2000. PMID: 11028557 - Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats.
Wan Y, Freeswick PD, Khemlani LS, Kispert PH, Wang SC, Su GL, Billiar TR. Wan Y, et al. Infect Immun. 1995 Jul;63(7):2435-42. doi: 10.1128/iai.63.7.2435-2442.1995. Infect Immun. 1995. PMID: 7790054 Free PMC article. - Expression of CD14 protein and its gene in liver sinusoidal endothelial cells during endotoxemia.
Gong JP, Dai LL, Liu CA, Wu CX, Shi YJ, Li SW, Li XH. Gong JP, et al. World J Gastroenterol. 2002 Jun;8(3):551-4. doi: 10.3748/wjg.v8.i3.551. World J Gastroenterol. 2002. PMID: 12046090 Free PMC article. - Primary structure of rat CD14 and characteristics of rat CD14, cytokine, and NO synthase mRNA expression in mononuclear phagocyte system cells in response to LPS.
Takai N, Kataoka M, Higuchi Y, Matsuura K, Yamamoto S. Takai N, et al. J Leukoc Biol. 1997 Jun;61(6):736-44. doi: 10.1002/jlb.61.6.736. J Leukoc Biol. 1997. PMID: 9201265
Cited by
- Augmenter of liver regeneration (ALR) is a novel biomarker of hepatocellular stress/inflammation: in vitro, in vivo and in silico studies.
Vodovotz Y, Prelich J, Lagoa C, Barclay D, Zamora R, Murase N, Gandhi CR. Vodovotz Y, et al. Mol Med. 2013 Jan 22;18(1):1421-9. doi: 10.2119/molmed.2012.00183. Mol Med. 2013. PMID: 23073658 Free PMC article. - Aloin Preconditioning Attenuates Hepatic Ischemia/Reperfusion Injury via Inhibiting TLR4/MyD88/NF-_κ_B Signal Pathway In Vivo and In Vitro.
Du Y, Qian B, Gao L, Tan P, Chen H, Wang A, Zheng T, Pu S, Xia X, Fu W. Du Y, et al. Oxid Med Cell Longev. 2019 Nov 20;2019:3765898. doi: 10.1155/2019/3765898. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827674 Free PMC article. - Comparative proteomic analysis of the effects of high-concentrate diet on the hepatic metabolism and inflammatory response in lactating dairy goats.
Duanmu Y, Cong R, Tao S, Tian J, Dong H, Zhang Y, Ni Y, Zhao R. Duanmu Y, et al. J Anim Sci Biotechnol. 2016 Feb 6;7:5. doi: 10.1186/s40104-016-0065-0. eCollection 2016. J Anim Sci Biotechnol. 2016. PMID: 26855776 Free PMC article. - Ablation of Aquaporin-9 Ameliorates the Systemic Inflammatory Response of LPS-Induced Endotoxic Shock in Mouse.
Tesse A, Gena P, Rützler M, Calamita G. Tesse A, et al. Cells. 2021 Feb 18;10(2):435. doi: 10.3390/cells10020435. Cells. 2021. PMID: 33670755 Free PMC article. - Effect of CD14 promoter polymorphism and H. pylori infection and its clinical outcomes on circulating CD14.
Karhukorpi J, Yan Y, Niemela S, Valtonen J, Koistinen P, Joensuu T, Saikku P, Karttunen R. Karhukorpi J, et al. Clin Exp Immunol. 2002 May;128(2):326-32. doi: 10.1046/j.1365-2249.2002.01837.x. Clin Exp Immunol. 2002. PMID: 11985523 Free PMC article.
References
- Arditi M, Zhou J, Torres M, Durden D L, Stins M, Kim K S. Lipopolysaccharide stimulates the tyrosine phosphorylation of mitogen-activated protein kinase p44, p42, and p41 in vascular endothelial cells in a soluble CD14-dependent manner. J Immunol. 1995;155:3994–4003. - PubMed
- Baumann H, Morella K K, Wong G H W. TNFα, IL-1β, and hepatocyte growth factor cooperate in stimulating specific acute-phase plasma protein gene in rat hepatoma cells. J Immunol. 1993;151:4248–4255. - PubMed
- Bellezzo J M, Britton R S, Bacon B R, Fox E S. LPS-mediated NF-kappa beta activation in rat Kupffer cells can be induced independently of CD14. Am J Physiol. 1996;270:G956–G961. - PubMed
- Boggaram V, Mendelson C R. Transcriptional regulation of the gene coding the major surfactant protein (SPA) in rabbit fetal lung. J Biol Chem. 1988;263:19060–19065. - PubMed
- Burgmann H, Winkler S, Locker G J, Presterl E, Laczika K, Staudinger T, Knapp S, Thalhammer F, Wenisch C, Zedwitz-Liebenstein K, Frass M, Graninger W. Increased serum concentration of soluble CD14 is a prognostic marker in gram-positive sepsis. Clin Immunol Immunopathol. 1996;80:307–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials