Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism - PubMed (original) (raw)
Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism
M C Kullberg et al. Infect Immun. 1998 Nov.
Abstract
Mice rendered deficient in interleukin-10 (IL-10) by gene targeting (IL-10(-/-) mice) develop chronic enterocolitis resembling human inflammatory bowel disease (IBD) when maintained in conventional animal facilities. However, they display a minimal and delayed intestinal inflammatory response when reared under specific-pathogen-free (SPF) conditions, suggesting the involvement of a microbial component in pathogenesis. We show here that experimental infection with a single bacterial agent, Helicobacter hepaticus, induces chronic colitis in SPF-reared IL-10(-/-) mice and that the disease is accompanied by a type 1 cytokine response (gamma interferon [IFN-gamma], tumor necrosis factor alpha, and nitric oxide) detected by restimulation of spleen and mesenteric lymph node cells with a soluble H. hepaticus antigen (Ag) preparation. In contrast, wild-type (WT) animals infected with the same bacteria did not develop disease and produced IL-10 as the dominant cytokine in response to Helicobacter Ag. Strong H. hepaticus-reactive antibody responses as measured by Ag-specific total immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, IgG3, and IgA were observed in both WT and IL-10(-/-) mice. In vivo neutralization of IFN-gamma or IL-12 resulted in a significant reduction of intestinal inflammation in H. hepaticus-infected IL-10(-/-) mice, suggesting an important role for these cytokines in the development of colitis in the model. Taken together, these microbial reconstitution experiments formally establish that a defined bacterial agent can serve as the immunological target in the development of large bowel inflammation in IL-10(-/-) mice and argue that in nonimmunocompromised hosts IL-10 stimulated in response to intestinal flora is important in preventing IBD.
Figures
FIG. 1
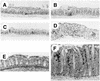
Immunostaining for CD3+ cells of Bouin’s-fixed cecum and colon of IL-10−/− and WT mice, all with hematoxylin counterstain. (A to C) Ceca of representative uninfected WT (A), infected WT (B), and uninfected IL-10−/− (C) mice at 4 weeks, showing few CD3+ cells. (D) Cecum of infected IL-10−/− mouse at 4 weeks, with many CD3+ cells and marked thickening (hyperplasia) of the epithelium. (E) Colon of uninfected IL-10−/− mouse at 4 weeks, with few CD3+ cells in the lamina propria. (F) Colon of infected IL-10−/− mouse at 4 weeks, with many CD3+ cells in lamina propria and marked diffuse epithelial hyperplasia. Magnifications: (A to D) ×75, (E and F) ×150.
FIG. 2
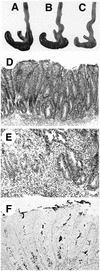
Gross and microscopic intestinal pathology of _H. hepaticus_-infected mice. (A to C) Gross pathology of portions of proximal large bowels of 4-week-infected WT (A), uninfected IL-10−/− (B), and 4-week-infected IL-10−/− (C) mice. Note the smaller, paler cecum of the infected IL-10−/− mouse; histologically, the mouse had typhlitis and diffuse hyperplasia. (D) Diffuse cecal hyperplasia in infected IL-10−/− mouse at 9 weeks, showing marked inflammation in lamina propria and submucosa. H&E stain. (E) Severe focal atypical hyperplasia in cecum of infected IL-10−/− mouse at 16 weeks. The muscularis mucosa is disrupted by atypical hyperplastic cells. H&E stain. (F) Steiner stain of cecum from infected IL-10−/− mouse at 4 weeks, showing numerous bacteria within epithelial crypts. Magnifications, ×75 (D) and ×150 (E and F).
FIG. 3
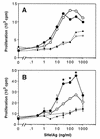
SHelAg-induced proliferative responses of cells from _H. hepaticus_-infected WT and IL-10−/− mice. Spleen cells (2.5 × 106/ml) (A) and MLN cells (1.5 × 106/ml) (B) from 4-week-infected (solid lines) and uninfected (dotted lines) WT (○) and IL-10−/− (•) mice were stimulated in vitro with various doses of SHelAg. Cultures were pulsed with [3H]thymidine after 48 h and harvested 18 h later. Spleen cell results represent the mean values for three individual infected and a pool of two uninfected mice per group. MLN results represent means ± SD of duplicate cultures of pools of the same mice shown in A. Data for one of six experiments (using 4-, 6-, 10-, or 11-week-infected mice) with similar results are shown. An asterisk indicates a significant difference (P < 0.05) in proliferative response between infected WT and IL-10−/− mice.
FIG. 4
SHelAg-specific Ab in sera of _H. hepaticus_-infected WT and IL-10−/− mice. Sera from uninfected (▵, ▴) and 16-week-infected (○, •) WT (open symbols) and IL-10−/− (closed symbols) mice were analyzed for levels of Ab (total IgG, IgG1, IgG2a, IgG2b, IgG3, and IgA) reactive to SHelAg by ELISA as described in Materials and Methods. Each dot represents the mean optical density (OD) value obtained from two separate pools of sera per group tested in duplicate (three females and three males in each pool).
FIG. 5
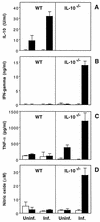
SHelAg-induced cytokine responses of spleen cells from _H. hepaticus_-infected WT and IL-10−/− mice. Spleen cells (5 × 106/ml) from uninfected (Uninf.) and 9-week-infected (Inf.) WT and IL-10−/− mice were stimulated with medium alone (□) or 1 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), TNF-α (C), and NO (D) were measured in 72-h supernatants. Bars represent means ± SD of values obtained from two separate pools of mice per group tested in duplicate (three females and three males in each pool). Data for one of seven experiments (using 4-, 6-, 9-, 10-, or 11-week-infected mice) with similar results are shown.
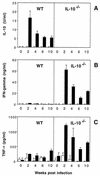
FIG. 6
SHelAg-induced cytokine responses of MLN cells from _H. hepaticus_-infected WT and IL-10−/− mice. MLN cells (3 × 106/ml) from uninfected and 2-, 4-, 6-, and 10-week-infected WT and IL-10−/− mice were stimulated with medium alone (□) or 1 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), and TNF-α (C) were measured in 72-h supernatants. Bars represent means ± SD of duplicate ELISA values from a pool of two to three infected WT and three to four infected IL-10−/− mice per time point. Cytokine levels for the 0-week postinfection time point have been calculated after pooling data from three separate determinations for uninfected mice assayed in parallel with the infected mice at 4, 6, and 10 weeks postinfection.
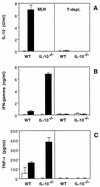
FIG. 7
T-cell depletion (T-depl.) of MLN populations from _H. hepaticus_-infected mice abolishes SHelAg-induced cytokine secretion. Total (left) and anti-Thy1.2-plus-complement-treated (right) MLN cells from 4-week-infected WT and IL-10−/− mice were cultured with medium alone (□) or 0.3 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), and TNF-α (C) were measured in 72-h supernatants. Bars represent means ± SD of duplicate ELISA values from a pool of three mice per group.
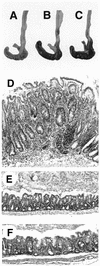
FIG. 8
Treatment with anti-IFN-γ or anti-IL-12 inhibits development of _H. hepaticus_-induced colitis in IL-10−/− mice. IL-10−/− mice were treated every 3 to 4 days with 1 mg of neutralizing MAb to IFN-γ or IL-12 or with a control MAb starting 1 day prior to H. hepaticus inoculation, and tissues were examined 4 weeks later. Shown are gross pathology (A to C) and histology (D to F) of ceca from 4-week-infected IL-10−/− mice treated with control (A and D), anti-IFN-γ (B and E), or anti-IL-12 (C and F) MAb. Note the small, pale cecum from the mouse treated with control MAb (A) and much less inflammation and epithelial hyperplasia in mice receiving anticytokine MAb (E to F). (D to F) Formalin-fixed tissues stained with H&E; magnification, ×75.
Similar articles
- Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation.
Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A. Kullberg MC, et al. Infect Immun. 2001 Jul;69(7):4232-41. doi: 10.1128/IAI.69.7.4232-4241.2001. Infect Immun. 2001. PMID: 11401959 Free PMC article. - Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope.
Kullberg MC, Andersen JF, Gorelick PL, Caspar P, Suerbaum S, Fox JG, Cheever AW, Jankovic D, Sher A. Kullberg MC, et al. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15830-5. doi: 10.1073/pnas.2534546100. Epub 2003 Dec 12. Proc Natl Acad Sci U S A. 2003. PMID: 14673119 Free PMC article. - Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis.
Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Kullberg MC, et al. J Exp Med. 2002 Aug 19;196(4):505-15. doi: 10.1084/jem.20020556. J Exp Med. 2002. PMID: 12186842 Free PMC article. - Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer.
Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Fox JG, et al. Mucosal Immunol. 2011 Jan;4(1):22-30. doi: 10.1038/mi.2010.61. Epub 2010 Oct 13. Mucosal Immunol. 2011. PMID: 20944559 Free PMC article. Review. - Genetic and environmental factors shape the host response to Helicobacter hepaticus: insights into IBD pathogenesis.
Jeffery R, Ilott NE, Powrie F. Jeffery R, et al. Curr Opin Microbiol. 2022 Feb;65:145-155. doi: 10.1016/j.mib.2021.10.012. Epub 2021 Dec 6. Curr Opin Microbiol. 2022. PMID: 34883389 Review.
Cited by
- The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health.
Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE Jr, Yamashita PS, Fox JH, Reber SO, Brenner LA, Hoisington AJ, Postolache TT, Kinney KA, Marciani D, Hernandez M, Hemmings SM, Malan-Muller S, Wright KP, Knight R, Raison CL, Rook GA. Lowry CA, et al. Curr Environ Health Rep. 2016 Sep;3(3):270-86. doi: 10.1007/s40572-016-0100-5. Curr Environ Health Rep. 2016. PMID: 27436048 Free PMC article. Review. - Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation.
Boulard O, Kirchberger S, Royston DJ, Maloy KJ, Powrie FM. Boulard O, et al. J Exp Med. 2012 Jul 2;209(7):1309-24. doi: 10.1084/jem.20120239. Epub 2012 Jun 25. J Exp Med. 2012. PMID: 22734048 Free PMC article. - Consequences of immunopathology for pathogen virulence evolution and public health: malaria as a case study.
Long GH, Graham AL. Long GH, et al. Evol Appl. 2011 Mar;4(2):278-91. doi: 10.1111/j.1752-4571.2010.00178.x. Evol Appl. 2011. PMID: 25567973 Free PMC article. - Intestinal Inflammation and Altered Gut Microbiota Associated with Inflammatory Bowel Disease Render Mice Susceptible to Clostridioides difficile Colonization and Infection.
Abernathy-Close L, Barron MR, George JM, Dieterle MG, Vendrov KC, Bergin IL, Young VB. Abernathy-Close L, et al. mBio. 2021 Jun 29;12(3):e0273320. doi: 10.1128/mBio.02733-20. Epub 2021 Jun 15. mBio. 2021. PMID: 34126769 Free PMC article. - Helicobacter pylori and Crohn's disease: a retrospective single-center study from China.
Xiang Z, Chen YP, Ye YF, Ma KF, Chen SH, Zheng L, Yang YD, Jin X. Xiang Z, et al. World J Gastroenterol. 2013 Jul 28;19(28):4576-81. doi: 10.3748/wjg.v19.i28.4576. World J Gastroenterol. 2013. PMID: 23901235 Free PMC article.
References
- Cao X, Shores E W, Hu-Li J, Anver M R, Kelsall B L, Russell S M, Drago J, Noguchi M, Grinberg A, Bloom E T, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. - PubMed
- Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous