Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats - PubMed (original) (raw)
Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats
J H Meijer et al. J Neurosci. 1998.
Abstract
The suprachiasmatic nuclei (SCN) of the hypothalamus contain a pacemaker that generates circadian rhythms in many functions. Light is the most important stimulus that synchronizes the circadian pacemaker to the environmental cycle. In this paper we have characterized the baseline neuronal firing patterns of the SCN as well as their response to light in freely moving rats. Multiunit and single-unit recordings showed that SCN neurons increase discharge during daytime and decrease discharge at night. Discharge levels of individual neurons that were followed throughout the circadian cycle appeared in phase with the population and were characterized by low discharge rates (often below 1 Hz), with a twofold increase during the day. The effect of light on the multiunit response was dependent on the duration of light exposure and on light intensity, with light thresholds of approximately 0.1 lux. The light response level showed a strong dependency on time of day, with large responsiveness at night and low responsiveness during day. At both phases of the circadian cycle, the response level could be raised by an increase in light intensity. Single-unit measurements revealed that the time-dependent light response of SCN neurons was present also at the level of single units. The results show that the basic light response characteristics that were observed at the multiunit level result from an integrated response of similarly behaving single units. Research at the single-unit level is therefore a useful approach for investigating the basic principles of photic entrainment.
Figures
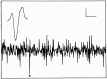
Fig. 1.
Representative example of an oscilloscope trace with multiunit activity. The spike indicated by a _dot_could be separated from the other spikes and could be recorded for several days. The inset shows the characteristic waveform of this spike. Horizontal calibration: 5 msec (oscilloscope trace) and 0.3 msec (inset). Vertical calibration: 10 μV throughout.
Fig. 2.
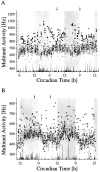
Long-term multiunit activity as a function of circadian time. Circadian time was determined on the basis of drinking actograms. The recordings were performed in constant darkness. The onset of drinking activity is the onset of the subjective night and corresponds with CT 12. The subjective night is shaded. Multiunit discharge inside (A) and outside (B) the SCN was counted every 10 sec.Dots represent mean values per minute. At the bottom of each figure, movement artifacts have been plotted. The gaps in the multiunit traces are the consequence of the exclusion of data bins in which movement artifacts occurred.
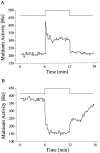
Fig. 3.
Multiunit light response of SCN neurons. The timing of the light pulse is indicated in the step diagram above the records. The light-activated (A) and light-suppressed (B) responses represent the mean of five and seven responses, respectively, obtained during two circadian cycles.
Fig. 4.
Responses to light pulses of different duration. Light responses of SCN neurons are sustained for 2, 6, and 30 min light pulses. The drop in discharge at the end of the 30 min light pulse is accidental. Discontinuity in the data, immediately after the onset of a light pulse, resulted from movement artifacts.
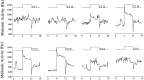
Fig. 5.
Light responses to increasing light intensities. Activated (A–C) and suppressed (D–F) response types with three to five traces averaged per light intensity. Intensities are indicated above the step diagram.
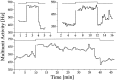
Fig. 6.
Light responses as a function of circadian time. Light intensity was set at 0.15 lux. Above the step diagram, circadian time (CT) of each response has been given. The change in magnitude of the light response throughout the circadian cycle is clearly visible, as is the circadian rhythm in basal MUA.
Fig. 7.
Light responses during long-term multiunit activity recordings inside (A) and outside (B) the SCN. The subjective night of the animal is shaded. Light pulses were given every hour for 6 min, with an intensity of 0.15 lux. In between the light presentations, the animal was in total darkness. The mean discharge rates per minute during the periods of darkness are indicated by a dot. The mean discharge rates per minute during the light intervals are indicated by triangles. Light responses marked by an_arrow_ are shown on a shorter time scale in Figure10.
Fig. 8.
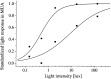
Averaged light sensitivity of activated SCN neurons during day (▪) and night (•). The maximum light response obtained in each animal was normalized to 1. The standardized light response is plotted against the light intensity and was obtained by taking the difference between baseline activity in the dark immediately before the light pulse, and discharge during light presentation. The difference was averaged for the mid subjective day ±3 hr (▪) and for mid subjective night ±3 hr (•). Data points were fitted with a Michaelis equation: y =_x_a/x_a+ b_a. The fitted parameters were_a = 0.6, b = 9 lux and_a = 1.2, b = 0.5 lux for the day and the night, respectively.
Fig. 9.
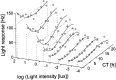
Light response of SCN neurons as a function of both light intensity and circadian time in a particular animal. The averaged response level was plotted against circadian time (assessed by the drinking rhythm) and against the logarithm of light intensity (in lux). The response level was determined by taking the difference between the dark discharge level before the light pulse and the discharge level during light and was averaged per 2 hr. For every light intensity used, the circadian variation in light response has been fitted by a free three-order polynomial, whereas the average is indicated by a dashed line, to enable visualization.
Fig. 10.
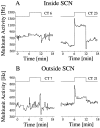
Light response inside (A) and outside (B) the SCN. Circadian time of the light pulses is specified above the step diagram. The long-term recording traces from which these examples are taken are given in Figure 7_A,B_. The timing of the light pulses is indicated by the arrows in Figure 7. Circadian rhythms in baseline discharge inside and outside the SCN are in opposite phase. Nevertheless, the rhythm in light response is similar inside and outside the SCN. Furthermore, it can be observed that light responses in the SCN were larger than outside the SCN, which was a general observation.
Fig. 11.
Simultaneous multiunit and single unit recording.A, Multiunit baseline discharge pattern (•) and discharge rate during hourly 6 min light presentation (▴). The_trace_ at the bottom indicates the occurrence of movement artifacts. B, Single-unit baseline discharge (•) varies between a mean of 0.05 Hz during night and 0.2 Hz during day. The light response level (▴) varies between mean levels of ∼1 Hz during night and 0.5 Hz during day. After a recording time of ∼36 hr the single unit was lost. Circadian time_12_ corresponds to the onset of drinking activity.
Fig. 12.
Long-term single-unit recording inside the SCN.A, Two circadian cycles of multiunit baseline activity indicated per minute in dots, with mean values of ∼1.5 Hz during night and 2.5 Hz during day. Responses to hourly light pulses of 6 min, 0.15 lux are indicated per minute by triangles_and vary between 16 Hz during night and 8 Hz during day. Circadian time_12 corresponds to the onset of drinking activity of the animal. B, Example of a light response during daytime at a different scale. The example is taken from the recording depicted in_A_ and represents the mean response during mid subjective day (CT 6 ± 2 hr). C, Light response during mid subjective night (CT 18 ± 2 hr).
Similar articles
- Circadian rhythm in light response in suprachiasmatic nucleus neurons of freely moving rats.
Meijer JH, Watanabe K, Détàri L, Schaap J. Meijer JH, et al. Brain Res. 1996 Nov 25;741(1-2):352-5. doi: 10.1016/s0006-8993(96)01091-8. Brain Res. 1996. PMID: 9001742 - Opposing effects of behavioural activity and light on neurons of the suprachiasmatic nucleus.
Schaap J, Meijer JH. Schaap J, et al. Eur J Neurosci. 2001 May;13(10):1955-62. doi: 10.1046/j.0953-816x.2001.01561.x. Eur J Neurosci. 2001. PMID: 11403689 - Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod.
Mrugala M, Zlomanczuk P, Jagota A, Schwartz WJ. Mrugala M, et al. Am J Physiol Regul Integr Comp Physiol. 2000 Apr;278(4):R987-94. doi: 10.1152/ajpregu.2000.278.4.R987. Am J Physiol Regul Integr Comp Physiol. 2000. PMID: 10749788 - Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network.
Meijer JH, Michel S, Vanderleest HT, Rohling JH. Meijer JH, et al. Eur J Neurosci. 2010 Dec;32(12):2143-51. doi: 10.1111/j.1460-9568.2010.07522.x. Eur J Neurosci. 2010. PMID: 21143668 Review. - Suprachiasmatic nucleus: the brain's circadian clock.
Gillette MU, Tischkau SA. Gillette MU, et al. Recent Prog Horm Res. 1999;54:33-58; discussion 58-9. Recent Prog Horm Res. 1999. PMID: 10548871 Review.
Cited by
- Fast delayed rectifier potassium current: critical for input and output of the circadian system.
Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Kudo T, et al. J Neurosci. 2011 Feb 23;31(8):2746-55. doi: 10.1523/JNEUROSCI.5792-10.2011. J Neurosci. 2011. PMID: 21414897 Free PMC article. - Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other's functioning?
Deboer T. Deboer T. Neurobiol Sleep Circadian Rhythms. 2018 Mar 1;5:68-77. doi: 10.1016/j.nbscr.2018.02.003. eCollection 2018 Jun. Neurobiol Sleep Circadian Rhythms. 2018. PMID: 31236513 Free PMC article. Review. - Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver.
Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. Cailotto C, et al. PLoS One. 2009 May 21;4(5):e5650. doi: 10.1371/journal.pone.0005650. PLoS One. 2009. PMID: 19478857 Free PMC article. - Responses to Spatial Contrast in the Mouse Suprachiasmatic Nuclei.
Mouland JW, Stinchcombe AR, Forger DB, Brown TM, Lucas RJ. Mouland JW, et al. Curr Biol. 2017 Jun 5;27(11):1633-1640.e3. doi: 10.1016/j.cub.2017.04.039. Epub 2017 May 18. Curr Biol. 2017. PMID: 28528901 Free PMC article. - Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus.
Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Kudo T, et al. J Neurophysiol. 2013 Sep;110(5):1097-106. doi: 10.1152/jn.00114.2013. Epub 2013 Jun 5. J Neurophysiol. 2013. PMID: 23741043 Free PMC article.
References
- Besharse JC. The daily light-dark cycle and rhythmic metabolism in the photoreceptor-pigment epithelial complex. In: Osborne N, Chader G, editors. Progress in retinal research, Vol 1. Pergamon; Oxford: 1982. pp. 81–124.
- Block GD, Khalsa SBS, McMahon DG, Michel S, Guesz M. Biological clocks in the retina: cellular mechanisms of biological timekeeping. Int Rev Cytol. 1993;146:83–144. - PubMed
- Brann MR, Cohen LV. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987;235:585–587. - PubMed
- Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–91. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources