Mitochondrial adenine nucleotide translocase is modified oxidatively during aging - PubMed (original) (raw)
Mitochondrial adenine nucleotide translocase is modified oxidatively during aging
L J Yan et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The purpose of this study was to test the hypothesis that elevation in protein oxidative damage during the aging process is a targeted rather than a stochastic phenomenon. Oxidative damage to proteins in mitochondrial membranes in the flight muscles of the housefly, manifested as carbonyl modifications, was detected immunochemically with anti-dinitrophenyl antibodies. Adenine nucleotide translocase (ANT) was found to be the only protein in the mitochondrial membranes exhibiting a detectable age-associated increase in carbonyls. The age-related elevation in ANT carbonyl content was correlated with a corresponding loss in its functional activity. Senescent flies that had lost the ability to fly exhibited a relatively higher degree of ANT oxidation and a greater loss of functional activity than their cohorts of the same age that were still able to fly. Exposure of flies to 100% oxygen resulted in an increase in the level of ANT carbonyl content and a loss in its activity. In vitro treatment of mitochondria with a system that generated hydroxyl free radicals caused an increase in ANT carbonyl level and a decrease in ANT exchange activity. ANT was also the only mitochondrial membrane protein exhibiting adducts of the lipid peroxidation product 4-hydroxynonenal. Results of this study indicate that proteins in mitochondrial membranes are modified selectively during aging.
Figures
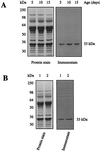
Figure 1
Immunochemical detection of protein carbonyls in mitochondrial membranes from flight muscles of houseflies. DNPH-treated proteins (10 μg) were electrophoresed on SDS/PAGE (10% resolving gel) under reducing conditions and transferred to Immobilon-P membrane. Oxidized proteins were detected immunochemically as described in the text. Lane 1, protein standard markers; lane 2, proteins without DNPH treatment; lanes 3–5, 5-, 10-, and 15-day-old fly mitochondrial proteins, respectively, treated with DNPH. A 33-kDa protein in the membranes exhibited a strong immunoreaction for the carbonyl groups.
Figure 2
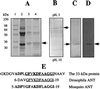
Purification and identification of the 33-kDa protein as housefly mitochondrial ANT. (A) Lane 1, standard markers; lane 2, crude membrane proteins; lane 3, eluate from DEAE column; lane 4, eluate from carboxymethyl column. (B) Reverse isoelectric focusing analysis of the eluate from carboxymethyl column. Arrow indicates the focused 33-kDa protein. (C) The focused band in B was excised from reverse isoelectric focusing gel and analyzed further by SDS/PAGE. Arrow indicates the purified protein. (D) The purified protein was immunochemically confirmed to be carbonylated. A, B, and C are protein stained by Coomassie blue; D is immunostain. (E) A computer-assisted search from protein database. The underlined amino acids show the N-terminal amino acid sequence homology in mitochondrial ANT from the housefly and Drosophila; the bold amino acids show homology with the mosquito.
Figure 3
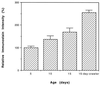
Immunochemical estimation of the carbonyl content of the 33-kDa protein in housefly at different ages. Presented values are mean ± SD of relative densitometric determinations in four independent experiments that used flies hatched on different dates. Immunostain intensity of the protein from 5-day-old flies was assigned an arbitrary value of 100; the ratio of 10- or 15-day-old flies and crawlers to the 5-day-old flies is reported. The increase in ANT carbonyl content between each age group was statistically significant.
Figure 4
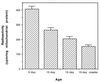
Exchange activity of mitochondrial ANT in the flight muscles of the houseflies at different ages. See text for the detailed method. Values are the mean ± SD of four experiments that used flies hatched on different dates. The drop in exchange activity between each age group was statistically significant.
Figure 5
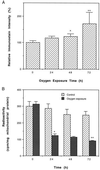
Effects of hyperoxia on ANT carbonyl formation and ANT activity. (A) Immunochemical estimation of ANT carbonyls in houseflies exposed to 100% ambient oxygen. Shown are relative densitometric values expressed as mean ± SD of four independent experiments. Immunostain intensity of the control was assigned an arbitrary value of 100, the ratio of oxygen-treated to control is reported. Flies that were 9 days old were exposed to 100% oxygen for indicated periods. (∗, P < 0.05 vs. 0 h; ∗∗, P < 0.01 vs. 48 h.) The changes between 0 and 24 h and between 24 and 48 h were not statistically significant. (B) Effect of 100% oxygen on the exchange activity of mitochondrial ANT. Shown are the mean ± SD of four independent determinations. (∗, P < 0.001 vs. control; ∗∗, P < 0.005 vs. 48 h.) The changes between each group of the control (no oxygen exposure) and between 24 and 48 h of oxygen exposure were not statistically significant.
Figure 6
Metal-catalyzed oxidation of mitochondrial membrane proteins and inactivation of ANT. Mitochondria were incubated with 1 mM vanadyl sulfate and 1 mM H2O2, followed by either membrane isolation or ANT activity measurements. (A) Immunochemical detection of ANT without oxidation (lane 1) and with oxidation (lane 2). (B) Increase of total mitochondrial membrane protein carbonyls after oxidation. Carbonyls were determined spectrophotometrically. (C) Loss of ANT activity caused by metal-catalyzed oxidation. Whole mitochondria were incubated with various concentrations of the oxidants (vanadyl/H2O2 = 1:1) before ANT activity measurements. For both B and C, values are the mean ± SD of four independent determinations that used 10-day-old flies hatched on different dates.
Figure 7
Immunochemical detection of HNE adducts in ANT. HNE detection was performed as described (23). Rabbit anti-HNE antibody (IgG) was diluted to 1:10,000 in Tris-buffered saline containing 0.1% Tween-20 with overnight exposure of the blot at 4°C. Goat anti-rabbit IgG conjugated with horseradish peroxidase was diluted 1:50,000 in Tris-buffered saline containing 0.1% Tween-20 with 3 h incubation at room temperature. (A) Age-associated changes in HNE level in ANT. (B) Comparison of HNE adducts in ANT between 15-day-old flies (lane 1) and crawlers (lane 2).
Similar articles
- Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator.
Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, Kroemer G. Vieira HL, et al. Cell Death Differ. 2000 Dec;7(12):1146-54. doi: 10.1038/sj.cdd.4400778. Cell Death Differ. 2000. PMID: 11175251 Review. - Oxidative damage during aging targets mitochondrial aconitase.
Yan LJ, Levine RL, Sohal RS. Yan LJ, et al. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11168-72. doi: 10.1073/pnas.94.21.11168. Proc Natl Acad Sci U S A. 1997. PMID: 9326580 Free PMC article. - Human FAM173A is a mitochondrial lysine-specific methyltransferase that targets adenine nucleotide translocase and affects mitochondrial respiration.
Małecki JM, Willemen HLDM, Pinto R, Ho AYY, Moen A, Eijkelkamp N, Falnes PØ. Małecki JM, et al. J Biol Chem. 2019 Aug 2;294(31):11654-11664. doi: 10.1074/jbc.RA119.009045. Epub 2019 Jun 18. J Biol Chem. 2019. PMID: 31213526 Free PMC article.
Cited by
- Rapid Repression of ADP Transport by Palmitoyl-CoA Is Attenuated by Exercise Training in Humans: A Potential Mechanism to Decrease Oxidative Stress and Improve Skeletal Muscle Insulin Signaling.
Ludzki A, Paglialunga S, Smith BK, Herbst EA, Allison MK, Heigenhauser GJ, Neufer PD, Holloway GP. Ludzki A, et al. Diabetes. 2015 Aug;64(8):2769-79. doi: 10.2337/db14-1838. Epub 2015 Apr 6. Diabetes. 2015. PMID: 25845660 Free PMC article. - Focus on: The cardiovascular system: what did we learn from the French (Paradox)?
Mochly-Rosen D, Zakhari S. Mochly-Rosen D, et al. Alcohol Res Health. 2010;33(1-2):76-86. Alcohol Res Health. 2010. PMID: 23579938 Free PMC article. Review. - Mitochondrial physiology in the major arbovirus vector Aedes aegypti: substrate preferences and sexual differences define respiratory capacity and superoxide production.
Soares JB, Gaviraghi A, Oliveira MF. Soares JB, et al. PLoS One. 2015 Mar 24;10(3):e0120600. doi: 10.1371/journal.pone.0120600. eCollection 2015. PLoS One. 2015. PMID: 25803027 Free PMC article. - Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster.
Sun J, Folk D, Bradley TJ, Tower J. Sun J, et al. Genetics. 2002 Jun;161(2):661-72. doi: 10.1093/genetics/161.2.661. Genetics. 2002. PMID: 12072463 Free PMC article. - Mitochondrial channelopathies in aging.
Pi Y, Goldenthal MJ, Marín-García J. Pi Y, et al. J Mol Med (Berl). 2007 Sep;85(9):937-51. doi: 10.1007/s00109-007-0190-5. Epub 2007 Apr 11. J Mol Med (Berl). 2007. PMID: 17426949 Review.
References
- Harman D. J Gerontol. 1956;11:298–300. - PubMed
- Beckman K B, Ames B N. Physiol Rev. 1998;78:547–581. - PubMed
- Harman D. Drugs Aging. 1993;3:60–80. - PubMed
- Beckman K B, Ames B N. J Biol Chem. 1997;272:19633–19636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous