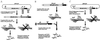Noncytopathic Sindbis virus RNA vectors for heterologous gene expression - PubMed (original) (raw)
Noncytopathic Sindbis virus RNA vectors for heterologous gene expression
E V Agapov et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Infection of vertebrate cells with alphaviruses normally leads to prodigious expression of virus-encoded genes and a dramatic inhibition of host protein synthesis. Recombinant Sindbis viruses and replicons have been useful as vectors for high level foreign gene expression, but the cytopathic effects of viral replication have limited their use to transient studies. We recently selected Sindbis replicons capable of persistent, noncytopathic growth in BHK cells and describe here a new generation of Sindbis vectors useful for long-term foreign gene expression based on such replicons. Foreign genes of interest as well as the dominant selectable marker puromycin N-acteyltransferase, which confers resistance to the drug puromycin, were expressed as subgenomic transcripts of noncytopathic replicons or defective-interfering genomes complemented in trans by a replicon. Based on these strategies, we developed vectors that can be initiated via either RNA or DNA transfection and analyzed them for their level and stability of foreign gene expression. Noncytopathic Sindbis vectors express reasonably high levels of protein in nearly every cell. These vectors should prove to be flexible tools for the rapid expression of heterologous genes under conditions in which cellular metabolism is not perturbed, and we illustrate their utility with a number of foreign proteins.
Figures
Figure 1
Noncytopathic SIN vectors. (A) SINrep19 RNA, the double subgenomic noncytopathic RNA vector, transcribes foreign genes and the pac gene from negative-strand templates by using separate subgenomic promoters (promoter positions are indicated by arrows in both DNA and RNA constructs). (B) A bipartite noncytopathic RNA vector relies on SINrep18 to support the replication of a defective, interfering SIN genome containing a gene of interest. The 5′ terminus of the DI RNA has a tRNA Asp sequence (20). (C) SINrep21 is a DNA vector for the SINrep19 replicon. After DNA transfection, replicon RNAs were transcribed via nuclear enzymes and transported to the cytoplasm. Replicons initiate autonomous replication and express the pac gene and foreign genes of interest. Run-off refers to convenient sites for run-off transcription; MCS, multiple-cloning sites.
Figure 2
Stability of β-gal expression by noncytopathic replicons. During the course of cell passage (1:10, approximately every 48 h), a portion of cells were seeded in separate wells and stained with X-gal as described in Materials and Methods. Stability of β-gal expressing cloned cell lines was assessed by using clones selected for high level expression. The percentage of β-gal-negative cells was calculated by microscopic examination of several random fields.
Figure 3
Expression of model proteins via noncytopathic vectors. Cells were selected after transfection with SINrep19/GFP (A), SINrep19/_lac_Z (B), SINrep19/CD46 (C and E), or SINrep18 (D and F). All of the cells were representative populations selected for purR as described in the text. Cells were prepared by X-gal staining (B) or immunofluorescent staining for CD46 (C and D). (E and F) show the condition of cells 54 h after infection with MV [multiplicity of infection (moi) 0.1].
Figure 4
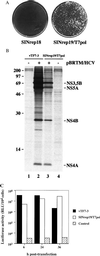
Functional expression of T7 RNA polymerase. (A) Mengo virus plaques after transfection of control BHK cells (SINrep18) or T7-expressing BHK cells (SINrep19/T7pol) with a T7-driven functional clone of Mengo virus. (B) Expression of the HCV polyprotein with (lanes 2 and 3) or without (lanes 1 and 4) transfection of pBRTM/HCV827–3011. Lanes 1 and 2 were derived from cells infected with vTF7–3, whereas lanes 3 and 4 represent cells containing SINrep19/T7pol. (C) Expression of luciferase after transfection of pEMCLucβgAn into cells expressing T7 RNA polymerase via vTF7–3, SINrep19/T7pol, or not expressing T7 RNA polymerase (control). Lysates were prepared at 6, 24, or 36 h after transfection. This experiment is representative of three independent experiments.
Comment in
- RNA virus vectors: where are we and where do we need to go?
Palese P. Palese P. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):12750-2. doi: 10.1073/pnas.95.22.12750. Proc Natl Acad Sci U S A. 1998. PMID: 9788984 Free PMC article. Review. No abstract available.
Similar articles
- Replicon vectors derived from Sindbis virus and Semliki forest virus that establish persistent replication in host cells.
Perri S, Driver DA, Gardner JP, Sherrill S, Belli BA, Dubensky TW Jr, Polo JM. Perri S, et al. J Virol. 2000 Oct;74(20):9802-7. doi: 10.1128/jvi.74.20.9802-9807.2000. J Virol. 2000. PMID: 11000258 Free PMC article. - Alphavirus cDNA-based expression vectors: effects of RNA transcription and nuclear export.
Boorsma M, Saudan P, Pfruender H, Bailey JE, Schlesinger S, Renner WA, Bachmann MF. Boorsma M, et al. Biotechnol Bioeng. 2003 Mar 5;81(5):553-62. doi: 10.1002/bit.10496. Biotechnol Bioeng. 2003. PMID: 12514804 - Noncytopathic flavivirus replicon RNA-based system for expression and delivery of heterologous genes.
Varnavski AN, Khromykh AA. Varnavski AN, et al. Virology. 1999 Mar 15;255(2):366-75. doi: 10.1006/viro.1998.9564. Virology. 1999. PMID: 10069962 - Alphavirus-based expression vectors: strategies and applications.
Frolov I, Hoffman TA, Prágai BM, Dryga SA, Huang HV, Schlesinger S, Rice CM. Frolov I, et al. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11371-7. doi: 10.1073/pnas.93.21.11371. Proc Natl Acad Sci U S A. 1996. PMID: 8876142 Free PMC article. Review. - Recombinant Sindbis virus as an expression system for cell biology.
Piper RC, Slot JW, Li G, Stahl PD, James DE. Piper RC, et al. Methods Cell Biol. 1994;43 Pt A:55-78. doi: 10.1016/s0091-679x(08)60598-1. Methods Cell Biol. 1994. PMID: 7529867 Review. No abstract available.
Cited by
- Self-Replicating RNA Derived from the Genomes of Positive-Strand RNA Viruses.
Meyers G, Tews BA. Meyers G, et al. Methods Mol Biol. 2024;2786:25-49. doi: 10.1007/978-1-0716-3770-8_2. Methods Mol Biol. 2024. PMID: 38814389 - Construction of a mini-RNA replicon in Escherichia coli.
Kashiwagi A, Yomo T. Kashiwagi A, et al. Synth Biol (Oxf). 2023 Mar 3;8(1):ysad004. doi: 10.1093/synbio/ysad004. eCollection 2023. Synth Biol (Oxf). 2023. PMID: 36926307 Free PMC article. - The Nonmonotonic Dose Dependence of Protein Expression in Cells Transfected with Self-Amplifying RNA.
Tanimoto CR, Thurm AR, Brandt DS, Knobler CM, Gelbart WM. Tanimoto CR, et al. J Virol. 2022 Apr 13;96(7):e0185821. doi: 10.1128/jvi.01858-21. Epub 2022 Mar 16. J Virol. 2022. PMID: 35293773 Free PMC article. - High-Throughput Platform for Detection of Neutralizing Antibodies Using Flavivirus Reporter Replicon Particles.
Lücke AC, Vom Hemdt A, Wieseler J, Fischer C, Feldmann M, Rothenfusser S, Drexler JF, Kümmerer BM. Lücke AC, et al. Viruses. 2022 Feb 8;14(2):346. doi: 10.3390/v14020346. Viruses. 2022. PMID: 35215941 Free PMC article. - Rescue of Infectious Sindbis Virus by Yeast Spheroplast-Mammalian Cell Fusion.
Ding L, Brown DM, Glass JI. Ding L, et al. Viruses. 2021 Apr 1;13(4):603. doi: 10.3390/v13040603. Viruses. 2021. PMID: 33916100 Free PMC article.
References
- Strauss E G, Rice C M, Strauss J H. Virology. 1984;133:92–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources