Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO - PubMed (original) (raw)
Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO
S R Frank et al. Mol Biol Cell. 1998 Nov.
Free PMC article
Abstract
ARNO is a member of a family of guanine-nucleotide exchange factors with specificity for the ADP-ribosylation factor (ARF) GTPases. ARNO possesses a central catalytic domain with homology to yeast Sec7p and an adjacent C-terminal pleckstrin homology (PH) domain. We have previously shown that ARNO localizes to the plasma membrane in vivo and efficiently catalyzes ARF6 nucleotide exchange in vitro. In addition to a role in endocytosis, ARF6 has also been shown to regulate assembly of the actin cytoskeleton. To determine whether ARNO is an upstream regulator of ARF6 in vivo, we examined the distribution of actin in HeLa cells overexpressing ARNO. We found that, while expression of ARNO leads to disassembly of actin stress fibers, it does not result in obvious changes in cell morphology. However, treatment of ARNO transfectants with the PKC agonist phorbol 12-myristate 13-acetate results in the dramatic redistribution of ARNO, ARF6, and actin into membrane protrusions resembling lamellipodia. This process requires ARF activation, as actin rearrangement does not occur in cells expressing a catalytically inactive ARNO mutant. PKC phosphorylates ARNO at a site immediately C-terminal to its PH domain. However, mutation of this site had no effect on the ability of ARNO to regulate actin rearrangement, suggesting that phosphorylation of ARNO by PKC does not positively regulate its activity. Finally, we demonstrate that an ARNO mutant lacking the C-terminal PH domain no longer mediates cytoskeletal reorganization, indicating a role for this domain in appropriate membrane localization. Taken together, these data suggest that ARNO represents an important link between cell surface receptors, ARF6, and the actin cytoskeleton.
Figures
Figure 1
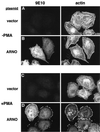
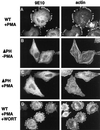
ARNO regulates cytoskeletal reorganization in HeLa cells treated with PMA. HeLa cells on coverslips were transiently transfected with empty vector (A and C) or vector encoding epitope-tagged ARNO (B and D). Cells were treated for 30 min without (A and B) or with 1 μM PMA (C and D), and then fixed in 2% formaldehyde. ARNO was detected by labeling with the monoclonal antibody 9E10 (anti-myc epitope) followed by Cy2-labeled donkey anti-mouse IgG (left panels). Cells were costained with rhodamine-labeled phalloidin to visualize filamentous actin (right panels).
Figure 2
ARNO and ARF6 colocalize in structures resembling lamellipodia after PMA treatment. HeLa cells cultured on coverslips were cotransfected with expression vectors encoding ARNO and HA-ARF6. Cells were treated 48 h later for 30 min without (A) or with 1 μM PMA (B), fixed, and double labeled with an affinity-purified polyclonal anti-ARNO antibody (ARNO) and a mouse anti-HA antibody (ARF6).
Figure 3
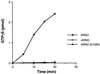
A Sec7 domain mutant is incapable of catalyzing nucleotide exchange on ARF6. Myristoylated ARF6 (1 μM) was incubated at 37°C in the absence (open circles) or presence of either 100 nM recombinant His6-ARNO (closed circles) or His6-E156K (open triangles) as described in MATERIALS AND METHODS. Data are the average of two simultaneous determinations and are representative of three separate experiments.
Figure 4
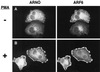
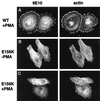
Catalytically inactive ARNO does not support cytoskeletal rearrangements in response to PMA treatment. HeLa cells transfected with plasmid encoding _myc_-tagged wild-type ARNO (A) or ARNO/E156K (B and C) were incubated in the presence (A and C) or absence (B) of PMA, fixed, and labeled for immunofluorescence as in Figure 1. Note the presence of intact stress fibers in panel B.
Figure 5
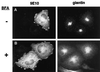
BFA does not inhibit actin reorganization in cells overexpressing ARNO. Cells transiently transfected with_myc_-tagged wild-type ARNO were incubated in medium without (A) or with (B) 10 μg/ml BFA for 40 min. PMA was included for the final 30 min of incubation. Cells were costained with the anti-myc antibody 9E10 (left panels) and rabbit polyclonal antibody recognizing the Golgi marker giantin (right panels).
Figure 6
PKC-dependent phosphorylation of ARNO does not positively regulate its ability to induce lamellipodia. A, HeLa cells expressing _myc_-tagged wild-type ARNO or ARNO(S392A) were metabolically labeled with [32P]orthophosphate for 4.5 h. During the final 30 min PMA (+) or DMSO (−) were added to culture medium. The cells were then lysed and immunoprecipitated with ARNO antiserum as described in MATERIALS AND METHODS. The resulting immune complexes were transferred to nitrocellulose membranes and analyzed by autoradiography (upper panel) followed by immunoblotting with anti-myc antibody (lower panel). B, Purified His6-ARNO was incubated with or without purified PKC for 30 min at 37°C, as described in MATERIALS AND METHODS. The PKC inhibitor BIM was included in one reaction at 10 μM. C, HeLa cells transfected with plasmid encoding_myc_-tagged wild-type ARNO or ARNO(S392A) were incubated with PMA, fixed, and labeled for immunofluorescence as in Figure 1. BIM was added to some cultures 10 min before the addition of PMA.
Figure 7
Cytoskeletal reorganization requires the ARNO PH domain, but not D3 phosphoinositides. HeLa cells transfected with plasmid encoding _myc_-tagged wild-type ARNO (A and D) or ARNOΔPH (B and C) were incubated with (A, C, and D) or without PMA (B), fixed, and labeled for immunofluorescence as in Figure 1. To some cultures (D) 100 nM wortmannin was added 15 min before the addition of PMA.
Similar articles
- ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling.
Li CC, Chiang TC, Wu TS, Pacheco-Rodriguez G, Moss J, Lee FJ. Li CC, et al. Mol Biol Cell. 2007 Nov;18(11):4420-37. doi: 10.1091/mbc.e07-02-0149. Epub 2007 Sep 5. Mol Biol Cell. 2007. PMID: 17804820 Free PMC article. - ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6.
Frank S, Upender S, Hansen SH, Casanova JE. Frank S, et al. J Biol Chem. 1998 Jan 2;273(1):23-7. doi: 10.1074/jbc.273.1.23. J Biol Chem. 1998. PMID: 9417041 - Regulation of ARNO nucleotide exchange by a PH domain electrostatic switch.
Santy LC, Frank SR, Hatfield JC, Casanova JE. Santy LC, et al. Curr Biol. 1999 Oct 21;9(20):1173-6. doi: 10.1016/S0960-9822(00)80019-6. Curr Biol. 1999. PMID: 10531036 - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review. - Arf GAPs as regulators of the actin cytoskeleton.
Randazzo PA, Inoue H, Bharti S. Randazzo PA, et al. Biol Cell. 2007 Oct;99(10):583-600. doi: 10.1042/bc20070034. Biol Cell. 2007. PMID: 17868031 Review.
Cited by
- Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D.
Santy LC, Casanova JE. Santy LC, et al. J Cell Biol. 2001 Aug 6;154(3):599-610. doi: 10.1083/jcb.200104019. Epub 2001 Jul 30. J Cell Biol. 2001. PMID: 11481345 Free PMC article. - The N-terminal coiled-coil domain of the cytohesin/ARNO family of guanine nucleotide exchange factors interacts with Galphaq.
Laroche G, Giguère PM, Dupré E, Dupuis G, Parent JL. Laroche G, et al. Mol Cell Biochem. 2007 Dec;306(1-2):141-52. doi: 10.1007/s11010-007-9564-9. Epub 2007 Sep 6. Mol Cell Biochem. 2007. PMID: 17846866 - Cytohesin-1 regulates beta-2 integrin-mediated adhesion through both ARF-GEF function and interaction with LFA-1.
Geiger C, Nagel W, Boehm T, van Kooyk Y, Figdor CG, Kremmer E, Hogg N, Zeitlmann L, Dierks H, Weber KS, Kolanus W. Geiger C, et al. EMBO J. 2000 Jun 1;19(11):2525-36. doi: 10.1093/emboj/19.11.2525. EMBO J. 2000. PMID: 10835351 Free PMC article. - Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant.
Al-Awar O, Radhakrishna H, Powell NN, Donaldson JG. Al-Awar O, et al. Mol Cell Biol. 2000 Aug;20(16):5998-6007. doi: 10.1128/MCB.20.16.5998-6007.2000. Mol Cell Biol. 2000. PMID: 10913182 Free PMC article. - The pleckstrin homology (PH) domain of the Arf exchange factor Brag2 is an allosteric binding site.
Jian X, Gruschus JM, Sztul E, Randazzo PA. Jian X, et al. J Biol Chem. 2012 Jul 13;287(29):24273-83. doi: 10.1074/jbc.M112.368084. Epub 2012 May 21. J Biol Chem. 2012. PMID: 22613714 Free PMC article.
References
- Achstetter T, Franzusoff A, Schekman R. SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J Biol Chem. 1988;263:11711–11717. - PubMed
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. - PubMed
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995;20:147–150. - PubMed
- Brewer CB. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. In: Roth MG, editor. Protein Expression in Animal Cells. Vol. 43. San Diego, CA: Academic Press; 1994. , 379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-38452/DK/NIDDK NIH HHS/United States
- P01 DK033506/DK/NIDDK NIH HHS/United States
- AI-32991/AI/NIAID NIH HHS/United States
- DK-33506/DK/NIDDK NIH HHS/United States
- P01 DK038452/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources