The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development - PubMed (original) (raw)
The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development
H Tian et al. Genes Dev. 1998.
Abstract
Mice lacking the hypoxia-inducible transcription factor EPAS1 die at mid-gestation. Despite normal morphological development of the circulatory system, EPAS1-deficient mice display pronounced bradycardia. In addition to the vascular endothelium, EPAS1 is expressed intensively in the organ of Zuckerkandl (OZ), the principle source of catecholamine production in mammalian embryos. EPAS1-deficient embryos contained substantially reduced catecholamine levels. Mid-gestational lethality was rescued by administration of the catecholamine precursor DOPS to pregnant females. We hypothesize that EPAS1 expressed in the OZ senses hypoxia during mid-gestational development and translates this signal into an altered pattern of gene expression, leading to increases in circulating catecholamine levels and proper cardiac function.
Figures
Figure 1
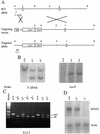
Mutation of the murine EPAS1 gene. (A) Schematic representations of wild-type (WT) EPAS1 allele showing locations of exons 2 and 3 and cleavage sites for _Eco_RI (E) and _Bam_HI (B) restriction enzymes, the targeting vector containing a viral thymidine kinase (TK) gene and the E. coli lacZ gene replacing exon 2 of the EPAS1 gene, and the product (targeted allele) of homologous recombination between the wild-type allele and vector. (B) Southern blot analysis of ES cell genomic DNA using a 5′ EPAS1 gene probe and a lacZ probe. (C) Inheritance of EPAS1 null allele by embryos of heterozygote crosses judged by PCR analysis of genomic DNA isolated from tails. (D) Blot analysis of total RNA (20 μg) isolated from E13.5 embryos using EPAS1 and β-actin cDNA probes.
Figure 2
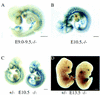
Vasculature and phenotype of EPAS1-deficient mice. (A–C) Surrogate EPAS1 expression in E9.0–9.5 and E10.5 embryos of the indicated EPAS1 genotypes was monitored by whole mount staining for β-galactosidase enzyme activity. (D) A living embryo heterozygous (+/−) for the EPAS1 null allele and a dead embryo homozygous (−/−) for the mutation were dissected on E13.5 and photographed. Congested blood cells are apparent within the liver of the −/− embryo. Bar, 100 μm (A–D).
Figure 3
Expression of EPAS1 in the sympathetic chain and OZ. (A–D) EPAS1 expression was monitored in histological sections and whole-mount embryos by β-gal staining. Sections derived from E11.5 (A), E12.5 (B), and E13.5 (C) embryos homozygous for a _lacZ_-tagged EPAS1 allele revealed expression in cells of the sympathetic chain (SC), endothelial cells of the dorsal aorta (DA) and renal artery (RA), and chromaffin cells of the OZ and adrenal gland (A). No expression was detected in cells of the cardinal vein (CV) or neural tube (NT). Bar, 10 μm. (D) Whole-mount staining of a heterozygous embryo on E15.5 revealed intense β-galactosidase expression in the OZ and lower levels of expression in the adrenal (A), both of which are located near the kidney (K) and testis (T). Bar, 50 μm.
Figure 4
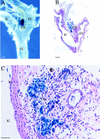
EPAS1 expression in adult carotid body. (A) Whole-mount staining of adult (age, 2.5 months) heterozygote showing intense β-gal expression in the carotid body (CB), which is located at the bifurcation of the common carotid artery (CC) into the internal carotid (IC) and external carotid (EC) arteries. (B,C) Adjacent histological sections revealing expression in the endothelial and type I cells of the carotid body. Bar, 100 μm (B); 50 μm (C).
Similar articles
- Coexpression of endothelial PAS protein 1 with essential angiogenic factors suggests its involvement in human vascular development.
Favier J, Kempf H, Corvol P, Gasc JM. Favier J, et al. Dev Dyn. 2001 Nov;222(3):377-88. doi: 10.1002/dvdy.1207. Dev Dyn. 2001. PMID: 11747073 - Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice.
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Scortegagna M, et al. Nat Genet. 2003 Dec;35(4):331-40. doi: 10.1038/ng1266. Epub 2003 Nov 9. Nat Genet. 2003. PMID: 14608355 - Cloning and expression pattern of EPAS1 in the chicken embryo. Colocalization with tyrosine hydroxylase.
Favier J, Kempf H, Corvol P, Gasc JM. Favier J, et al. FEBS Lett. 1999 Nov 26;462(1-2):19-24. doi: 10.1016/s0014-5793(99)01476-3. FEBS Lett. 1999. PMID: 10580084 - EPAS1/HIF-2α is a driver of mammalian pexophagy.
Schönenberger MJ, Krek W, Kovacs WJ. Schönenberger MJ, et al. Autophagy. 2015;11(6):967-9. doi: 10.1080/15548627.2015.1045180. Autophagy. 2015. PMID: 25997392 Free PMC article. Review. - [A new transcriptional factor--EPAS1].
Chen W. Chen W. Sheng Li Ke Xue Jin Zhan. 2000 Oct;31(4):334-6. Sheng Li Ke Xue Jin Zhan. 2000. PMID: 11372426 Review. Chinese. No abstract available.
Cited by
- Hypoxia-inducible factor as an angiogenic master switch.
Hashimoto T, Shibasaki F. Hashimoto T, et al. Front Pediatr. 2015 Apr 24;3:33. doi: 10.3389/fped.2015.00033. eCollection 2015. Front Pediatr. 2015. PMID: 25964891 Free PMC article. Review. - HIF hydroxylase pathways in cardiovascular physiology and medicine.
Bishop T, Ratcliffe PJ. Bishop T, et al. Circ Res. 2015 Jun 19;117(1):65-79. doi: 10.1161/CIRCRESAHA.117.305109. Circ Res. 2015. PMID: 26089364 Free PMC article. Review. - Tailless and hypoxia inducible factor-2α cooperate to sustain proangiogenic states of retinal astrocytes in neonatal mice.
Duan LJ, Jiang Y, Shi Y, Fong GH. Duan LJ, et al. Biol Open. 2023 Jan 1;12(1):bio059684. doi: 10.1242/bio.059684. Epub 2023 Jan 10. Biol Open. 2023. PMID: 36625299 Free PMC article. - Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing.
Webb JD, Coleman ML, Pugh CW. Webb JD, et al. Cell Mol Life Sci. 2009 Nov;66(22):3539-54. doi: 10.1007/s00018-009-0147-7. Epub 2009 Sep 12. Cell Mol Life Sci. 2009. PMID: 19756382 Free PMC article. Review. - Expression of HIF-1α is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer.
Jögi A, Ehinger A, Hartman L, Alkner S. Jögi A, et al. PLoS One. 2019 Dec 10;14(12):e0226150. doi: 10.1371/journal.pone.0226150. eCollection 2019. PLoS One. 2019. PMID: 31821370 Free PMC article.
References
- Copp AJ. Death before birth: Clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. - PubMed
- Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem. 1992;58:1538–1546. - PubMed
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & Dev. 1994;8:1897–1909. - PubMed
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci. 1997;94:4273–4278. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials