Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection - PubMed (original) (raw)
Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection
N Bayer et al. J Virol. 1998 Dec.
Abstract
Bafilomycin A1 (baf), a specific inhibitor of vacuolar proton ATPases, is commonly employed to demonstrate the requirement of low endosomal pH for viral uncoating. However, in certain cell types baf also affects the transport of endocytosed material from early to late endocytic compartments. To characterize the endocytic route in HeLa cells that are frequently used to study early events in viral infection, we used 35S-labeled human rhinovirus serotype 2 (HRV2) together with various fluid-phase markers. These virions are taken up via receptor-mediated endocytosis and undergo a conformational change to C-antigenic particles at a pH of <5.6, resulting in release of the genomic RNA and ultimately in infection (E. Prchla, E. Kuechler, D. Blaas, and R. Fuchs, J. Virol. 68:3713-3723, 1994). As revealed by fluorescence microscopy and subcellular fractionation of microsomes by free-flow electrophoresis (FFE), baf arrests the transport of all markers in early endosomes. In contrast, the microtubule-disrupting agent nocodazole was found to inhibit transport by accumulating marker in endosomal carrier vesicles (ECV), a compartment intermediate between early and late endosomes. Accordingly, lysosomal degradation of HRV2 was suppressed, whereas its conformational change and infectivity remained unaffected by this drug. Analysis of the subcellular distribution of HRV2 and fluid-phase markers in the presence of nocodazole by FFE revealed no difference from the control incubation in the absence of nocodazole. ECV and late endosomes thus have identical electrophoretic mobilities, and intraluminal pHs of <5.6 and allow uncoating of HRV2. As bafilomycin not only dissipates the low endosomal pH but also blocks transport from early to late endosomes in HeLa cells, its inhibitory effect on viral infection could in part also be attributed to trapping of virus in early endosomes which might lack components essential for uncoating. Consequently, inhibition of viral uncoating by bafilomycin cannot be taken to indicate a low pH requirement only.
Figures
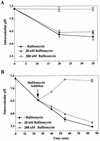
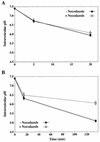
FIG. 1
baf raises the pH of early and late endosomes in HeLa cells. (A) HeLa cells were preincubated for 30 min at 37°C without or with 20 nM or 200 nM baf. They were then incubated for 5 min in medium containing 6 mg of FITC-dextran and 1 mg of Cy5-dextran per ml and subsequently chased in dextran-free medium in the absence or presence of baf. Cells were immediately cooled, washed with PBS, and analyzed by flow cytometry. (B) HeLa cells were continuously labeled with 6 mg of FITC-dextran and 1 mg Cy5-dextran per ml for 25 min. Cells were then further incubated in fresh medium without or with 20 nM or 200 nM baf (arrow). The average pH of labeled endocytic compartments was calculated with a pH calibration curve (see Materials and Methods). Times shown in both panels represent total time from the addition of FITC-dextran and Cy5-dextran. Values shown are means and standard deviations from two experiments (each sample was analyzed eight times).
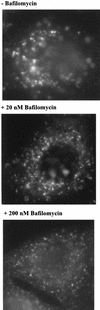
FIG. 2
Influence of baf on endocytic transport of FITC-dextran as analyzed by fluorescence microscopy. HeLa cells grown on chamber slides were preincubated without or with baf (20 nM or 200 nM) in L15 medium for 30 min at 37°C. Ten milligrams of FITC-dextran per ml was added, and endosomes were labeled for 25 min at 37°C. Cells were cooled, rinsed with PBS, fixed with 4% paraformaldehyde, quenched with 50 mM NH4Cl in PBS, and viewed with an Olympus fluorescence microscope.
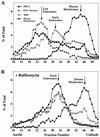
FIG. 3
baf inhibits delivery of HRV2 and fluid-phase markers to late endocytic compartments. HeLa cells preincubated without (A) or with (B) 200 nM baf (30 min, 37°C) were divided into three aliquots each. (i) Late endosomes were labeled with FITC-dextran (20 mg/ml; 3-min pulse, 12-min chase in marker-free medium); HRP (10 mg/ml) was then added for 3 min at 37°C to label the early endosomes. (ii) 35S-labeled HRV2 (106 cpm) was internalized for 20 min at 34°C. (iii) 3H-labeled poliovirus (6.5 × 105 cpm) was bound to HeLa cells for 60 min at 4°C to label plasma membranes. The aliquots of the labeled cells were cooled and combined, washed with PBS containing 10 mM EDTA to remove plasma membrane bound HRV2, and homogenized in TEA-sucrose buffer (see Materials and Methods). Microsomes were analyzed by FFE. A total of 92 fractions were collected, and protein content, radioactivity, and HRP activity, were determined. Data are expressed as the percentage of the total amount recovered after FFE.
FIG. 4
baf prevents infection of HRV2. HeLa cells were preincubated in infection medium (with or without 20 or 200 nM baf) for 30 min at 34°C. HRV2 was added at an MOI of 500. At 4 h postinfection, the medium was replaced with fresh methionine-free medium containing 200 μCi of [35S]methionine, and incubation was continued overnight. Cells were collected by centrifugation and lysed, and newly synthesized viral proteins were immunoprecipitated with anti-HRV2 antiserum. Viral proteins were analyzed by polyacrylamide gel electrophoresis followed by autoradiography. Where indicated, baf was present throughout the experiment.
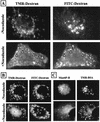
FIG. 5
Effect of nocodazole on fluid-phase marker transport in HeLa cells. (A) Cells on chamber slides were incubated without or with 20 μM nocodazole in serum-free MEM (30 min, 37°C). Late endosomes were labeled by pulse (10 min)-chase (15 min) with 10 mg of FITC-dextran per ml followed by early endosome labeling with 10 mg of TMR-dextran per ml for 5 min, and the cells were prepared for fluorescence microscopy. Where indicated, nocodazole was present throughout. FITC and TMR fluorescence images of the same cells are shown. Arrows indicate ECV; arrowheads indicate colocalization of markers in early endosomes. (B) Late endosomes were labeled by pulse (10 min)-chase (10 min) with 10 mg of FITC-dextran per ml followed by TMR-dextran (10 mg/ml) internalization for 5 min. Cells were rapidly cooled and incubated in the absence (upper panels) or presence of nocodazole for 1 h at 4°C before being warmed to 37°C for 5 min. In the absence of nocodazole, both markers colocalized in late endosomes (arrowheads, upper panels). When cells were treated with nocodazole after labeling of early and late endosomes, transfer of TMR-dextran to late, FITC-labeled endosomes (arrows, lower right panel) was arrested in EVC (arrows, lower left panel). (C) Cells were preincubated without or with nocodazole as in panel A, followed by internalization of 10 mg of TMR-BSA per ml for 15 min and a 10-min chase in marker-free medium (with or without nocodazole). Cells were cooled and fixed, and Man6P-R was detected by indirect immunofluorescence. In control cells, TMR-BSA was mainly detected in Man6P-R-positive compartments (late endosomes [arrowheads, upper panels]). Disruption of microtubules arrested the transport of TMR-BSA in ECV (arrows, lower right panel), resulting in a considerable reduction of colocalization with Man6P-R-containing structures (late endosomes [arrows, lower left panel]).
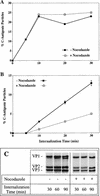
FIG. 6
Effect of nocodazole on the pH of endocytic compartments. HeLa cells were preincubated (with or without 20 μM nocodazole) in DMEM for 30 min at 37°C. (A) Endosomes were labeled with FITC (6 mg/ml)- and Cy5 (1 mg/ml)-dextran in DMEM for 5 min and then chased for 15 min in marker-free medium. (B) Cells were labeled for 15 min with FITC-dextran and Cy5-dextran (concentrations as in panel A) and chased for 120 min. The fluorescence intensity of the internalized markers was determined by flow cytometry. The average pH of endocytic compartments was calculated as described in Materials and Methods. The times shown in both panels represent the total time from the addition of FITC-dextran and Cy5-dextran. The values shown represent the means and standard deviations of two independent experiments (each analyzed eight times).
FIG. 7
HRV2 is converted to C-antigenic particles but lysosomal degradation is prevented in nocodazole-treated cells. (A and B) HeLa cells were preincubated (with or without 20 μM nocodazole) for 30 min at 34°C in infection medium and infected with [35S]HRV2 (105 cpm) at 34°C. Aliquots were removed at 5, 10, 20, and 30 min. Samples were rapidly cooled, cells were pelleted, plasma membrane bound virus was removed with PBS–10 mM EDTA, and cell pellets were lysed in RIPA buffer. Lysates (A) and cell supernatants (B) were subjected to immunoprecipitation with monoclonal antibody 2G2, which is specific for C-antigenic particles, and polyclonal antiserum. (C) Cells were preincubated and infected as in panel A for 30 min. Unbound virus was removed with PBS, and incubation was continued with or without nocodazol for the times indicated. Virus was recovered from cell lysates by immunoprecipitation with 2G2. Viral proteins were analyzed by SDS-gel electrophoresis followed by autoradiography.
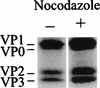
FIG. 8
HeLa cells are infected in the presence of nocodazole. Cells were preincubated in infection medium with or without 20 μM nocodazole for 30 min at 34°C. HRV2 was added at an MOI of 500. At 4 h postinfection, the medium was replaced with fresh, methionine-free medium containing 200 μCi of [35S]methionine, and incubation was continued overnight. Cells were collected by centrifugation and then lysed; newly synthesized viral proteins were next immunoprecipitated with anti-HRV2 antiserum. Viral proteins were analyzed by polyacrylamide gel electrophoresis followed by autoradiography. Where indicated, nocodazole was present throughout the experiment.
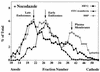
FIG. 9
ECV comigrate with late endosomes upon FFE separation. HeLa cells were preincubated in serum-free MEM with or without (Fig. 3) 20 μM nocodazole for 30 min at 37°C. Aliquots of the cell suspension were labeled with FITC-transferrin (20 μg/ml, 30 min at 37°C), HRP (10 mg/ml, 3 min, 37°C), and 35S-labeled HRV2 (106 cpm, 30 min at 34°C), respectively. Microsomes were prepared and analyzed by FFE. Data are expressed as the percentage of the total amount of the respective marker recovered after FFE.
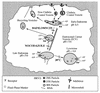
FIG. 10
Schematic representation of stages of endocytic traffic in HeLa cells blocked by baf and nocodazole. baf inhibits budding of ECV, leading to the accumulation of cargo in early endosomes, whereas nocodazol depolymerizes microtubules, thus inhibiting the transport of ECV to late endosomes, but it has only a minor effect on recycling to the plasma membrane.
Similar articles
- Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump.
van Weert AW, Dunn KW, Geuze HJ, Maxfield FR, Stoorvogel W. van Weert AW, et al. J Cell Biol. 1995 Aug;130(4):821-34. doi: 10.1083/jcb.130.4.821. J Cell Biol. 1995. PMID: 7642700 Free PMC article. - Uncoating of human rhinovirus serotype 2 from late endosomes.
Prchla E, Kuechler E, Blaas D, Fuchs R. Prchla E, et al. J Virol. 1994 Jun;68(6):3713-23. doi: 10.1128/JVI.68.6.3713-3723.1994. J Virol. 1994. PMID: 8189509 Free PMC article. - Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature.
Baravalle G, Schober D, Huber M, Bayer N, Murphy RF, Fuchs R. Baravalle G, et al. Cell Tissue Res. 2005 Apr;320(1):99-113. doi: 10.1007/s00441-004-1060-x. Epub 2005 Feb 16. Cell Tissue Res. 2005. PMID: 15714281 - Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2.
Zhao Z, Qin P, Huang YW. Zhao Z, et al. Cell Calcium. 2021 Mar;94:102360. doi: 10.1016/j.ceca.2021.102360. Epub 2021 Jan 23. Cell Calcium. 2021. PMID: 33516131 Free PMC article. Review. - Uncoating of human rhinoviruses.
Fuchs R, Blaas D. Fuchs R, et al. Rev Med Virol. 2010 Sep;20(5):281-97. doi: 10.1002/rmv.654. Rev Med Virol. 2010. PMID: 20629045 Review.
Cited by
- Neuromasts and Olfactory Organs of Zebrafish Larvae Represent Possible Sites of SARS-CoV-2 Pseudovirus Host Cell Entry.
Choi SSA, Chan HH, Chan CM, Wang X, Webb SE, Leung KW, Tsim KWK, Miller AL. Choi SSA, et al. J Virol. 2022 Dec 21;96(24):e0141822. doi: 10.1128/jvi.01418-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448804 Free PMC article. - Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling.
Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, Buganim Y, Schroeder A, Langer R, Anderson DG. Sahay G, et al. Nat Biotechnol. 2013 Jul;31(7):653-8. doi: 10.1038/nbt.2614. Epub 2013 Jun 23. Nat Biotechnol. 2013. PMID: 23792629 Free PMC article. - Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis.
Snyers L, Zwickl H, Blaas D. Snyers L, et al. J Virol. 2003 May;77(9):5360-9. doi: 10.1128/jvi.77.9.5360-5369.2003. J Virol. 2003. PMID: 12692238 Free PMC article. - Endocytic host cell machinery plays a dominant role in intracellular trafficking of incoming human immunodeficiency virus type 1 in human placental trophoblasts.
Vidricaire G, Imbeault M, Tremblay MJ. Vidricaire G, et al. J Virol. 2004 Nov;78(21):11904-15. doi: 10.1128/JVI.78.21.11904-11915.2004. J Virol. 2004. PMID: 15479831 Free PMC article. - Differentiation-dependent rearrangements of actin filaments and microtubules hinder apical endocytosis in urothelial cells.
Tratnjek L, Romih R, Kreft ME. Tratnjek L, et al. Histochem Cell Biol. 2017 Aug;148(2):143-156. doi: 10.1007/s00418-017-1566-4. Epub 2017 Apr 10. Histochem Cell Biol. 2017. PMID: 28397141
References
- Anbari M, Root K V, Van Dyke R W. Role of Na,K-ATPase in regulating acidification of early rat liver endocytic vesicles. Hepatology. 1994;19:1034–1043. - PubMed
- Balch W E, Rothman J E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985;240:413–425. - PubMed
- Bomsel M, Parton R, Kuznetsov S A, Schroer T A, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials