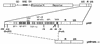Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery - PubMed (original) (raw)
Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery
R Zufferey et al. J Virol. 1998 Dec.
Abstract
In vivo transduction of nondividing cells by human immunodeficiency virus type 1 (HIV-1)-based vectors results in transgene expression that is stable over several months. However, the use of HIV-1 vectors raises concerns about their safety. Here we describe a self-inactivating HIV-1 vector with a 400-nucleotide deletion in the 3' long terminal repeat (LTR). The deletion, which includes the TATA box, abolished the LTR promoter activity but did not affect vector titers or transgene expression in vitro. The self-inactivating vector transduced neurons in vivo as efficiently as a vector with full-length LTRs. The inactivation design achieved in this work improves significantly the biosafety of HIV-derived vectors, as it reduces the likelihood that replication-competent retroviruses will originate in the vector producer and target cells, and hampers recombination with wild-type HIV in an infected host. Moreover, it improves the potential performance of the vector by removing LTR sequences previously associated with transcriptional interference and suppression in vivo and by allowing the construction of more-stringent tissue-specific or regulatable vectors.
Figures
FIG. 1
Structure of SIN HIV-derived vectors. A schematic representation of an HIV-1 vector with enlarged 3′ LTR to show the binding sites for differents transcription factors on U3 is shown (not to scale). Although the 3′ LTR is depicted, the nucleotide numbering refers to the cap site at the beginning of R as +1 as for a 5′ LTR. Position −418 is the 5′ limit of all deletions; positions −78, −45, −36, and −18 indicate the 3′ limits of the different deletions described in the text. The deletion generating the SIN-18 vector created a novel _Bgl_II site. Details on the nuclear factors binding U3 can be found in references , , and and references therein. SD, splice donor; RRE, Rev-response element; SA, splice acceptor. The GenBank accession number for the wild-type 3′ LTR is M1991.
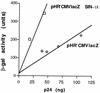
FIG. 2
Expression of a lacZ transgene delivered by SIN or full-length LTR vectors. 293T cells were transduced with equal volumes (200 μl) of two HR′CMVlacZSIN-18 or four HR′CMV lacZ vector stocks. Titers (TU per nanogram of p24) were similar for all stocks. β-Gal activity (in arbitrary units) at 48 h postinfection is plotted against the amount of p24 in vector stocks. Cells transduced with SIN-18 vectors express more than twice as much β-Gal per nanogram of p24 than cells transduced with full-length LTR vectors.
FIG. 3
Northern blot analysis of vector-derived transcripts in transduced HeLa cells. Total RNA was extracted from HeLa cells transduced with an RRLPGK-GFP vector (lane 1) or with its SIN versions SIN-18 (lane 2), SIN-36 (lane 3), SIN-45 (lane 4), and SIN-78 (lane 5). In lane 1, three bands with the sizes expected for the LTR-derived transcripts (unspliced and spliced) and the PGK-derived transcripts are visible. As expected for HeLa cells, transcription was initiated much more frequently at the internal PGK promoter than at the 5′ HIV-1 LTR. In lanes 2 to 5, transcripts derived from SIN vectors are 340 to 400 nucleotides smaller than the corresponding transcripts in lane 1. RNA initiated at the HIV-1 LTR is detectable in lane 5 but not in lanes 2 to 4. Positions of molecular size markers (in kilobases) are indicated on the right. Ψ, encapsidation signal; SD, splice donor; SA, splice acceptor.
FIG. 4
Activation pattern of HIV-1 vectors following infection of transduced SupT1 cells by HIV-1. Human lymphocytic SupT1 cells were transduced at a high multiplicity of infection by HIV-derived vectors carrying a PGK-GFP expression cassette and either a full-length LTR or the indicated U3 deletion construct. Six days later, the stably transduced cells were infected with VSV G-pseudotyped HIV-1 or were mock treated, and 48 h later they were analyzed by FACS for GFP fluorescence. Infection with HIV-1 strongly enhanced the expression of GFP in cells transduced by a vector with a full-length U3 LTR or the −78 deletion construct, while it had no effect on cells transduced with vectors having larger U3 deletions. The left and middle quadrants represent the fluorescence of cells not transduced and transduced by the GFP vector, respectively. The right quadrant includes cells with increased GFP expression upon infection by HIV-1. The increased expression of GFP indicates activation of vector transcription from the LTR and is due to translational readthrough of the PGK promoter sequence upstream of the GFP cDNA (see text). The increased in fluorescence intensity was 30-fold for cells transduced by the full-length LTR and 21-fold for those transduced by the SIN-78 vector. The HIV-1 had a deletion in the envelope gene and was thus limited to one round of infection. Similar patterns of Tat responsiveness were observed when HeLa-tat cells were transduced with the various vectors (not shown).
FIG. 5
In vivo transduction of GFP into neurons by SIN or full-length LTR vectors. HIV-1 vectors carrying a PGK-GFP expression cassette with the full-length U3 region (A and C) or the −18 deletion construct (B and D) were concentrated by ultracentrifugation and normalized for particle content prior to injection into the corpora striata of adult rats. One month after injection, brain sections were stained for immunoreactivity to the GFP protein. Both types of vectors transduced neurons very efficiently. The SIN vector often appeared to achieve a higher level of transgene expression. A representative section close to the injection site is shown for one of six injected striata per vector. Bars in panels A and C, 2 and 0.1 mm, respectively (magnifications are the same for panels B and D, respectively).
Similar articles
- A third-generation lentivirus vector with a conditional packaging system.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. Dull T, et al. J Virol. 1998 Nov;72(11):8463-71. doi: 10.1128/JVI.72.11.8463-8471.1998. J Virol. 1998. PMID: 9765382 Free PMC article. - Self-inactivating lentiviral vectors with U3 and U5 modifications.
Iwakuma T, Cui Y, Chang LJ. Iwakuma T, et al. Virology. 1999 Aug 15;261(1):120-32. doi: 10.1006/viro.1999.9850. Virology. 1999. PMID: 10441560 - Development of a self-inactivating lentivirus vector.
Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM. Miyoshi H, et al. J Virol. 1998 Oct;72(10):8150-7. doi: 10.1128/JVI.72.10.8150-8157.1998. J Virol. 1998. PMID: 9733856 Free PMC article. - Use of HIV as a gene transfer vector.
Pluta K, Kacprzak MM. Pluta K, et al. Acta Biochim Pol. 2009;56(4):531-95. Epub 2009 Nov 23. Acta Biochim Pol. 2009. PMID: 19936329 Review. - Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection.
Morris KV, Rossi JJ. Morris KV, et al. Curr HIV Res. 2004 Apr;2(2):185-91. doi: 10.2174/1570162043484906. Curr HIV Res. 2004. PMID: 15078182 Review.
Cited by
- Transgenic sheep generated by lentiviral vectors: safety and integration analysis of surrogates and their offspring.
Cornetta K, Tessanne K, Long C, Yao J, Satterfield C, Westhusin M. Cornetta K, et al. Transgenic Res. 2013 Aug;22(4):737-45. doi: 10.1007/s11248-012-9674-3. Epub 2012 Nov 23. Transgenic Res. 2013. PMID: 23180364 Free PMC article. - The determination of importance of sequences neighboring the Psi sequence in lentiviral vector transduction and packaging efficiency.
Kim SH, Jun HJ, Jang SI, You JC. Kim SH, et al. PLoS One. 2012;7(11):e50148. doi: 10.1371/journal.pone.0050148. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185560 Free PMC article. - The transformative potential of HSC gene therapy as a genetic medicine.
Sagoo P, Gaspar HB. Sagoo P, et al. Gene Ther. 2023 Apr;30(3-4):197-215. doi: 10.1038/s41434-021-00261-x. Epub 2021 May 26. Gene Ther. 2023. PMID: 34040164 Review. - Deoxycytidine-kinase knockdown as a novel myeloprotective strategy in the context of fludarabine, cytarabine or cladribine therapy.
Lachmann N, Czarnecki K, Brennig S, Phaltane R, Heise M, Heinz N, Kempf H, Dilloo D, Kaever V, Schambach A, Heuser M, Moritz T. Lachmann N, et al. Leukemia. 2015 Nov;29(11):2266-9. doi: 10.1038/leu.2015.108. Epub 2015 Apr 29. Leukemia. 2015. PMID: 25921248 No abstract available. - Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake.
Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C. Schindler AG, et al. J Neurosci. 2012 Dec 5;32(49):17582-96. doi: 10.1523/JNEUROSCI.3220-12.2012. J Neurosci. 2012. PMID: 23223282 Free PMC article.
References
- Chen B F, Hsieh C L, Chen D S, Hwang L H. Improved gene expression by a U3-based retroviral vector. Biochem Biophys Res Commun. 1992;184:330–337. - PubMed
- Cullen B R, Lomedico P T, Ju G. Transcriptional interference in avian retroviruses—implications for the promoter insertion model of leukaemogenesis. Nature. 1984;307:241–245. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources