ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system - PubMed (original) (raw)
ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system
T L Yahr et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The exoenzyme S regulon is a set of coordinately regulated virulence genes of Pseudomonas aeruginosa. Proteins encoded by the regulon include a type III secretion and translocation apparatus, regulators of gene expression, and effector proteins. The effector proteins include two enzymes with ADP-ribosyltransferase activity (ExoS and ExoT) and an acute cytotoxin (ExoU). In this study, we identified ExoY as a fourth effector protein of the regulon. ExoY is homologous to the extracellular adenylate cyclases of Bordetella pertussis (CyaA) and Bacillus anthracis (EF). The homology among the three adenylate cyclases is limited to two short regions, one of which possesses an ATP-binding motif. In assays for adenylate cyclase activity, recombinant ExoY (rExoY) catalyzed the formation of cAMP with a specific activity similar to the basal activity of CyaA. In contrast to CyaA and EF, rExoY activity was not stimulated or activated by calmodulin. A 500-fold stimulation of activity was detected following the addition of a cytosolic extract from Chinese hamster ovary (CHO) cells. These results indicate that a eukaryotic factor, distinct from calmodulin, enhances rExoY catalysis. Site-directed mutagenesis of residues within the putative active site of ExoY abolished adenylate cyclase activity. Infection of CHO cells with ExoY-producing strains of P. aeruginosa resulted in the intracellular accumulation of cAMP. cAMP accumulation within CHO cells depended on an intact type III translocation apparatus, demonstrating that ExoY is directly translocated into the eukaryotic cytosol.
Figures
Figure 1
(A) Schematic representation of the ExoY, CyaA, and EF adenylate cyclases. The positions of conserved regions I, II, and III are shown. Solid boxes represent the calmodulin-binding domains of CyaA and EF. The glycine-rich repeats of CyaA (hatched), signal sequence (ss), and protective antigen-binding site of EF are labeled. (B)
pileup
alignment of conserved regions I and II. Residues within CyaA and EF that are homologous to ExoY are shaded. The position of ATP-binding site motif A and its consensus sequence are shown. Residues of ExoY altered by site-directed mutagenesis are indicated by arrows.
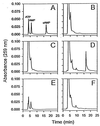
Figure 2
Reverse-phase HPLC analysis of cAMP production. The elution profile of ATP, AMP, and cAMP (30 nmol) is shown in A, standard reactions lacking enzyme in B, reactions containing a control extract from BL21pLysS in C, and 1.0 μM rExoY in D. ATP and cAMP (30 nmol) treated with 3′:5′-cyclic nucleotide phosphodiesterase is shown in E. A reaction identical to that seen in D treated with 5 μg of 3′:5′-cyclic nucleotide phosphodiesterase for 10 min at 30°C is shown in F.
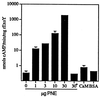
Figure 3
A eukaryotic factor stimulates rExoY adenylate cyclase activity. rExoY (1.0 μM) was assayed for adenylate cyclase activity in the presence or absence of PNE from CHO cells for 30 min under standard conditions. ∗ indicates that PNE was heated to 100°C for 5 min prior to addition to the assay mixture. Calmodulin (CaM, 10 μM) or 50 μg/ml BSA did not stimulate rExoY adenylate cyclase activity.
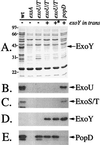
Figure 4
Extracellular protein profiles of ExoY-expressing strains. P. aeruginosa strains (as in Table 1; wt, PA103; exsA, PA103_exsA_∷Ω; exoU/T, PA103Δ_exoUexoT_∷Tc; popD, PA103_popD_∷Ω) were grown to an _OD_540 between 4.0 and 5.0, and extracellular fractions were prepared. A is a Coomassie-stained gel of concentrated supernatants. Molecular-mass markers (in kDa) are labeled on the left of A. B_–_E are immunoblots of gels identical to A probed with antisera against ExoU, ExoS/T, ExoY, and PopD, respectively. For ExoY expression in strain PA103, ExoY was amplified with its native promoter and cloned into pUCP18. ∗ denotes the expression of a catalytically (K81M) inactive form of ExoY.
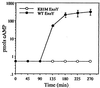
Figure 5
Kinetics of cAMP accumulation in infected CHO cells. CHO cells were infected with PA103Δ_exoUexoT_∷Tc expressing either native ExoY or catalytically inactive ExoYK81M. At the indicated times, lysate fractions were prepared and assayed for cAMP. Values are the average of three wells and reported as pmols of cAMP per 144 μg of cellular protein per well.
Similar articles
- Adenylate cyclase activity of Pseudomonas aeruginosa ExoY can mediate bleb-niche formation in epithelial cells and contributes to virulence.
Hritonenko V, Mun JJ, Tam C, Simon NC, Barbieri JT, Evans DJ, Fleiszig SM. Hritonenko V, et al. Microb Pathog. 2011 Nov;51(5):305-12. doi: 10.1016/j.micpath.2011.08.001. Epub 2011 Aug 9. Microb Pathog. 2011. PMID: 21843628 Free PMC article. - Bacterial Nucleotidyl Cyclases Activated by Calmodulin or Actin in Host Cells: Enzyme Specificities and Cytotoxicity Mechanisms Identified to Date.
Teixeira-Nunes M, Retailleau P, Comisso M, Deruelle V, Mechold U, Renault L. Teixeira-Nunes M, et al. Int J Mol Sci. 2022 Jun 16;23(12):6743. doi: 10.3390/ijms23126743. Int J Mol Sci. 2022. PMID: 35743184 Free PMC article. Review. - Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion.
Cowell BA, Evans DJ, Fleiszig SM. Cowell BA, et al. FEMS Microbiol Lett. 2005 Sep 1;250(1):71-6. doi: 10.1016/j.femsle.2005.06.044. FEMS Microbiol Lett. 2005. PMID: 16039071 - Pseudomonas aeruginosa exoenzyme Y directly bundles actin filaments.
Mancl JM, Suarez C, Liang WG, Kovar DR, Tang WJ. Mancl JM, et al. J Biol Chem. 2020 Mar 13;295(11):3506-3517. doi: 10.1074/jbc.RA119.012320. Epub 2020 Feb 4. J Biol Chem. 2020. PMID: 32019868 Free PMC article. - The Pseudomonas aeruginosa Exoenzyme Y: A Promiscuous Nucleotidyl Cyclase Edema Factor and Virulence Determinant.
Morrow KA, Frank DW, Balczon R, Stevens T. Morrow KA, et al. Handb Exp Pharmacol. 2017;238:67-85. doi: 10.1007/164_2016_5003. Handb Exp Pharmacol. 2017. PMID: 28181005 Free PMC article. Review.
Cited by
- A Simple Luminescent Adenylate-Cyclase Functional Assay for Evaluation of Bacillus anthracis Edema Factor Activity.
Israeli M, Rotem S, Elia U, Bar-Haim E, Cohen O, Chitlaru T. Israeli M, et al. Toxins (Basel). 2016 Aug 18;8(8):243. doi: 10.3390/toxins8080243. Toxins (Basel). 2016. PMID: 27548219 Free PMC article. - Translocon-independent intracellular replication by Pseudomonas aeruginosa requires the ADP-ribosylation domain of ExoS.
Hritonenko V, Evans DJ, Fleiszig SM. Hritonenko V, et al. Microbes Infect. 2012 Dec;14(15):1366-73. doi: 10.1016/j.micinf.2012.08.007. Epub 2012 Aug 30. Microbes Infect. 2012. PMID: 22981600 Free PMC article. - Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review.
Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP. Sawa T, et al. Crit Care. 2014 Dec 13;18(6):668. doi: 10.1186/s13054-014-0668-9. Crit Care. 2014. PMID: 25672496 Free PMC article. Review. - Catheter-associated urinary tract infection by Pseudomonas aeruginosa progresses through acute and chronic phases of infection.
Mekonnen SA, El Husseini N, Turdiev A, Carter JA, Belew AT, El-Sayed NM, Lee VT. Mekonnen SA, et al. Proc Natl Acad Sci U S A. 2022 Dec 13;119(50):e2209383119. doi: 10.1073/pnas.2209383119. Epub 2022 Dec 5. Proc Natl Acad Sci U S A. 2022. PMID: 36469780 Free PMC article. - Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system.
Dacheux D, Attree I, Schneider C, Toussaint B. Dacheux D, et al. Infect Immun. 1999 Nov;67(11):6164-7. doi: 10.1128/IAI.67.11.6164-6167.1999. Infect Immun. 1999. PMID: 10531282 Free PMC article.
References
- Bodey G P, Bolivar R, Fainstein V, Jadeja L. Rev Infect Dis. 1983;5:279–313. - PubMed
- Frank D W. Mol Microbiol. 1997;26:621–629. - PubMed
- Coburn J. Curr Top Microbiol Immunol. 1992;175:133–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources