HIV-1 variation diminishes CD4 T lymphocyte recognition - PubMed (original) (raw)
HIV-1 variation diminishes CD4 T lymphocyte recognition
G C Harcourt et al. J Exp Med. 1998.
Abstract
Effective long-term antiviral immunity requires specific cytotoxic T lymphocytes and CD4(+) T lymphocyte help. Failure of these helper responses can be a principle cause of viral persistence. We sought evidence that variation in HIV-1 CD4(+) T helper epitopes might contribute to this phenomenon. To determine this, we assayed fresh peripheral blood mononuclear cells from 43 asymptomatic HIV-1(+) patients for proliferative responses to HIV-1 antigens. 12 (28%) showed a positive response, and we went on to map dominant epitopes in two individuals, to p24 Gag restricted by human histocompatibility leukocyte antigen (HLA)-DR1 and to p17 Gag restricted by HLA-DRB52c. Nine naturally occurring variants of the p24 Gag epitope were found in the proviral DNA of the individual in whom this response was detected. All variants bound to HLA-DR1, but three of these peptides failed to stimulate a CD4(+) T lymphocyte line which recognized the index sequence. Antigenic variation was also detected in the p17 Gag epitope; a dominant viral variant present in the patient was well recognized by a specific CD4(+) T lymphocyte line, whereas several natural mutants were not. Importantly, variants detected at both epitopes also failed to stimulate fresh uncultured cells while index peptide stimulated successfully. These results demonstrate that variant antigens arise in HIV-1(+) patients which fail to stimulate the T cell antigen receptor of HLA class II-restricted lymphocytes, although the peptide epitopes are capable of being presented on the cell surface. In HIV-1 infection, naturally occurring HLA class II-restricted altered peptide ligands that fail to stimulate the circulating T lymphocyte repertoire may curtail helper responses at sites where variant viruses predominate.
Figures
Figure 1
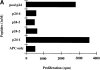
(A) Identification of HIV p24 Gag epitope recognized by T cell line 744. Proliferation assays were performed by culturing 5 × 104 T cells with 105 irradiated (30 Gy) autologous PBMCs as APCs with or without 1 μM peptide for 3 d. Peptides used to verify the specificity of the Gag response were as follows: p24-1, aa 133–153, PIVQNLQGQMVHQAISPRTL; p24-2, aa 143–163, VHQAISPRTL-MAWVKVVEEK; p24-3, aa 153–173, MAWVKVVEEKAF-SPEVIPMF; and p24-4, aa 163–183, AFSPEVIPMFSALSEGATQP. Cells were labeled with 1 μCi [3H]thymidine per well for 16 h, harvested, and counted on a beta counter. APC only, cpm obtained for T cells and APCs only. (B) Identification of the MHC haplotype that restricts presentation of antigen to T cell line 744. HLA class II–restricting elements were determined by proliferation assays using HLA-matched and mismatched PBMCs from healthy donors as APCs in the presence or absence of 1 μM peptide p24-1. HLA type of patient 744 (autol.APC) was A24/74;B35/44;DR1/12, DR52. Donor DR1/− was homozygous for DR1.
Figure 1
(A) Identification of HIV p24 Gag epitope recognized by T cell line 744. Proliferation assays were performed by culturing 5 × 104 T cells with 105 irradiated (30 Gy) autologous PBMCs as APCs with or without 1 μM peptide for 3 d. Peptides used to verify the specificity of the Gag response were as follows: p24-1, aa 133–153, PIVQNLQGQMVHQAISPRTL; p24-2, aa 143–163, VHQAISPRTL-MAWVKVVEEK; p24-3, aa 153–173, MAWVKVVEEKAF-SPEVIPMF; and p24-4, aa 163–183, AFSPEVIPMFSALSEGATQP. Cells were labeled with 1 μCi [3H]thymidine per well for 16 h, harvested, and counted on a beta counter. APC only, cpm obtained for T cells and APCs only. (B) Identification of the MHC haplotype that restricts presentation of antigen to T cell line 744. HLA class II–restricting elements were determined by proliferation assays using HLA-matched and mismatched PBMCs from healthy donors as APCs in the presence or absence of 1 μM peptide p24-1. HLA type of patient 744 (autol.APC) was A24/74;B35/44;DR1/12, DR52. Donor DR1/− was homozygous for DR1.
Figure 2
Naturally occurring variants of p24-1 Gag from patient 744. Proviral DNA was purified from PBMCs of patient 744 collected at intervals. The region of interest was amplified by nested PCR and sequenced. At least 20 clones were studied to identify any variants.
Figure 3
Proliferation of T cell line 744 to variant peptides of p24-1 Gag. Recognition of 16 variant peptides (see Table 3) of p24-1 Gag by T cell line 744 was assayed by proliferation, as described for Fig. 1_A_. APC only, background for T cells and APCs alone, i.e., SI = 1.
Figure 4
Naturally occurring variants of p17-3 Gag in patient 024. Viral DNA was purified from PBMCs of patient 024 at the time intervals stated. The region of p17-3 was amplified by PCR, cloned, and sequenced. At least 20 clones were sequenced to identify any variants.
Figure 5
Proliferation of T cell line 024 to variants of p17-3 Gag. Recognition of peptides by T cell line 024 was assessed by proliferation assays as described for Fig. 1_A_. Sequences of the p17 Gag variant peptides are as follows: p17-3, LRPGGKKKYKLKHIV; p17-3/1, LRPRGK-KRYKLKHIV; p17-3/2, LRPGGKKQYRLKHIV; p17-3/3, LRPGGKKKYQLKHIV; and p17-3/4, LRPGGKKKYALKHLI.
Similar articles
- Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants.
Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, et al. Klenerman P, et al. Nature. 1994 Jun 2;369(6479):403-7. doi: 10.1038/369403a0. Nature. 1994. PMID: 7515165 - Novel and promiscuous CTL epitopes in conserved regions of Gag targeted by individuals with early subtype C HIV type 1 infection from southern Africa.
Masemola AM, Mashishi TN, Khoury G, Bredell H, Paximadis M, Mathebula T, Barkhan D, Puren A, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM; HIVNET 028 Study Team. Masemola AM, et al. J Immunol. 2004 Oct 1;173(7):4607-17. doi: 10.4049/jimmunol.173.7.4607. J Immunol. 2004. PMID: 15383595 - HIV-1-specific CD4+ T-cell responses are not associated with significant viral epitope variation in persons with persistent plasma viremia.
Koeppe JR, Campbell TB, Rapaport EL, Wilson CC. Koeppe JR, et al. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):140-8. doi: 10.1097/01.qai.0000195608.32885.38. J Acquir Immune Defic Syndr. 2006. PMID: 16394844 - Major Histocompatibility Complex Binding, Eluted Ligands, and Immunogenicity: Benchmark Testing and Predictions.
Paul S, Grifoni A, Peters B, Sette A. Paul S, et al. Front Immunol. 2020 Feb 5;10:3151. doi: 10.3389/fimmu.2019.03151. eCollection 2019. Front Immunol. 2020. PMID: 32117208 Free PMC article. Review.
Cited by
- Selective pressures of HLA genotypes and antiviral therapy on human immunodeficiency virus type 1 sequence mutation at a population level.
Ahlenstiel G, Roomp K, Däumer M, Nattermann J, Vogel M, Rockstroh JK, Beerenwinkel N, Kaiser R, Nischalke HD, Sauerbruch T, Lengauer T, Spengler U; Kompetenznetz HIV/AIDS. Ahlenstiel G, et al. Clin Vaccine Immunol. 2007 Oct;14(10):1266-73. doi: 10.1128/CVI.00169-07. Epub 2007 Aug 22. Clin Vaccine Immunol. 2007. PMID: 17715334 Free PMC article. - Smarter vaccine design will circumvent regulatory T cell-mediated evasion in chronic HIV and HCV infection.
Moise L, Terry F, Gutierrez AH, Tassone R, Losikoff P, Gregory SH, Bailey-Kellogg C, Martin WD, De Groot AS. Moise L, et al. Front Microbiol. 2014 Oct 6;5:502. doi: 10.3389/fmicb.2014.00502. eCollection 2014. Front Microbiol. 2014. PMID: 25339942 Free PMC article. - Widespread adaptive evolution in the human immunodeficiency virus type 1 genome.
Yang W, Bielawski JP, Yang Z. Yang W, et al. J Mol Evol. 2003 Aug;57(2):212-21. doi: 10.1007/s00239-003-2467-9. J Mol Evol. 2003. PMID: 14562964 - Hepatitis C virus adaptation to T-cell immune pressure.
Plauzolles A, Lucas M, Gaudieri S. Plauzolles A, et al. ScientificWorldJournal. 2013;2013:673240. doi: 10.1155/2013/673240. Epub 2013 Mar 11. ScientificWorldJournal. 2013. PMID: 23554569 Free PMC article. Review. - Interplay between HIV-1 and Host Genetic Variation: A Snapshot into Its Impact on AIDS and Therapy Response.
Sampathkumar R, Shadabi E, Luo M. Sampathkumar R, et al. Adv Virol. 2012;2012:508967. doi: 10.1155/2012/508967. Epub 2012 May 16. Adv Virol. 2012. PMID: 22666249 Free PMC article.
References
- Lane HC, Masur H, Edgar G, Whalen AR, Rook AR, Fauci AS. Abnormalities of B cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. - PubMed
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. - PubMed
- Klenerman P, Meier UC, Phillips RE, McMichael AJ. The effects of natural altered peptide ligands on the whole blood cytotoxic T lymphocyte response to human immunodeficiency virus. Eur J Immunol. 1995;25:1927–1931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials