Nerve terminal currents induced by autoreception of acetylcholine release - PubMed (original) (raw)
Nerve terminal currents induced by autoreception of acetylcholine release
W M Fu et al. J Neurosci. 1998.
Abstract
The activation of autoreceptors is known to be important in the modulation of presynaptic transmitter secretion in peripheral and central neurons. Using whole-cell recordings made from the free growth cone of myocyte-contact motoneurons of Xenopus cell cultures, we have observed spontaneous nerve terminal currents (NTCs). These spontaneous NTCs are blocked by d-tubocurarine (d-TC) and alpha-bungarotoxin (alpha-BuTx), indicating that endogenously released acetylcholine (ACh) can produce substantial membrane depolarization in the nerve terminals. Local application of NMDA to the growth cone increased the frequency of spontaneous NTCs. When the electrical stimulations were applied at the soma to initiate evoked-release of ACh, evoked ACh-induced potentials were recorded in the nerve terminals, which were inhibited by d-TC and hexamethonium but not by atropine. Replacement of normal Ringer's solution with high-Mg2+, low-Ca2+ solution also reversibly inhibited evoked ACh-induced potentials. The possible regulatory role of presynaptic nicotinic autoreceptors on the synaptic transmission was also examined. When the innervated myocyte was whole-cell voltage-clamped to record synaptic currents, application of hexamethonium inhibited the amplitude of evoked synaptic currents at a higher degree than that of iontophoretic ACh-induced currents. Furthermore, hexamethonium markedly reduced the frequency of spontaneous synaptic currents at high-activity synapses. Pretreatment of neurons with alpha-BuTx also inhibited the evoked synaptic currents in manipulated synapses. These results suggest that ACh released spontaneously or by electrical stimulation may act on the presynaptic nicotinic autoreceptors of the same nerve terminals to produce membrane potential change and to regulate synaptic transmission.
Figures
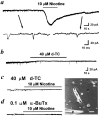
Fig. 1.
Spontaneous nerve terminal currents induced by the quantal release of ACh from the same nerve growth cone in 1-d-old_Xenopus_ cultures. Phase-contrast micrograph in the_bottom right corner_ shows the myocyte-contact neuron and the arrangement of the patch pipette for the whole-cell recording of nerve growth cone. Scale bar, 20 μm. N, Neuron;M, myocyte; P, patch pipette.a, Continuous trace depicts the membrane currents recorded from the nerve growth cone before and after local application of nicotine. Downward deflections are spontaneous NTCs (Vh = −60 mV, filtered at 150 Hz). Local perfusion with nicotine induced an inward current and reduced the frequency of NTCs. Samples of NTCs before and after nicotine treatment are shown_below_ at higher time resolution (filtered at 10 kHz).b, Application of d-TC inhibited the frequency of NTCs. Pretreatment of culture with d-TC (c) or α-BuTx (d) inhibited the appearance of NTCs and completely antagonized the nicotine-induced inward current.
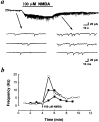
Fig. 2.
Potentiation of spontaneous nerve terminal currents by NMDA. a, Continuous trace_depicts the membrane currents recorded from the nerve growth cone before and after local application of NMDA. Downward deflections are spontaneous NTCs (Vh = −60 mV, filtered at 150 Hz). Local application of NMDA to the growth cone increased the frequency of NTCs. Samples of NTCs before and after NMDA treatment are shown_below at higher time resolution. The time course–response curves were shown in b. Each_curve_ connects data collected from one neuron.
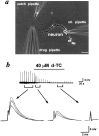
Fig. 3.
Nerve terminal potentials caused by the evoked release of ACh via the activation of nicotinic autoreceptors.a, Phase-contrast micrograph shows the cultured spinal neuron and the arrangement of the pipettes for whole-cell recording, electrical stimulation (sti. pipette), and drug application, respectively. Scale bar, 20 μm. b, The_trace_ represents the change in membrane potential of nerve growth cone induced by stimulating the soma at 0.1 Hz. The nerve growth cone was whole-cell current-clamped at resting membrane potential. Note that local perfusion with d-TC inhibited the potential change. The three to five superimposed potential signals were shown_below_ at higher time resolution.
Fig. 4.
Inhibition of evoked ACh-induced nerve terminal potential by hexamethonium and low Ca2+ medium. Nerve growth cone was whole-cell current-clamped at resting membrane potential. Evoked ACh-induced nerve terminal potentials were elicited by stimulating soma at 0.1 Hz. Note that hexamethonium (a), but not atropine (b), inhibited these depolarizing potentials. c, Replacement of normal Ringer’s solution with high-Mg2+, low-Ca2+ Ringer’s solution reversibly inhibited the evoked ACh-induced nerve terminal potentials.
Fig. 5.
Positive feedback regulation of ACh release by the activation of presynaptic nicotinic autoreceptors. The innervated myocyte was whole-cell voltage-clamped at −60 mV. Downward deflections indicated SSCs (filtered at 150 Hz). Application of nicotinic receptor antagonist hexamethonium markedly inhibited the spontaneous ACh release of the high-activity synapses (a) but only slightly affected that of low-activity synapses (b). c, A presynaptic neuron was stimulated at the soma to induce ESCs at 0.1 Hz. Note that application of hexamethonium inhibited the ESCs by 34%. d, Identical iontophoretic pulses of ACh at 0.5 Hz were applied to the surface of an isolated myocyte. Application of hexamethonium only slightly inhibited the iontophoretic ACh-induced currents.
Fig. 6.
Pretreatment of neurons with α-bungarotoxin inhibited evoked synaptic currents in manipulated synapses.a, The photograph shows that the ESCs were examined by moving a myoball (M1) to form a manipulated synapse with a naive neuron (N). After obtaining the control ESCs by stimulating (Sti.) the soma at 0.1 Hz, the M1 was removed away, and the culture was treated with α-BuTx (60 n
m
) for 20 min and then washed three times with plain Ringer’s solution. The second myoball (M2) from another culture dish, which was not treated with toxin, was then manipulated to form a synapse at the same site, and the evoked responses were measured again. Scale bar, 30 μm. b, The downward deflections in the middle trace indicated SSCs and ESCs (filled circles) (filtered at 150 Hz). α-BuTx treatment significantly inhibited the amplitude of ESCs but not that of SSCs. The_top panels_ show the representative superimposed SSC events during a 10 sec period, and the _bottom panels_show the four superimposed ESCs at higher time resolution.
Similar articles
- Nicotinic and muscarinic components in acetylcholine stimulation of porcine adrenal medullary cells.
Nassar-Gentina V, Catalán L, Luxoro M. Nassar-Gentina V, et al. Mol Cell Biochem. 1997 Apr;169(1-2):107-13. doi: 10.1023/a:1006867423715. Mol Cell Biochem. 1997. PMID: 9089637 - Activity-dependent modulation of developing neuromuscular synapses.
Poo MM. Poo MM. Adv Second Messenger Phosphoprotein Res. 1994;29:521-7. doi: 10.1016/s1040-7952(06)80033-9. Adv Second Messenger Phosphoprotein Res. 1994. PMID: 7848730 Review. - Regulatory role of ATP at developing neuromuscular junctions.
Fu WM. Fu WM. Prog Neurobiol. 1995 Sep;47(1):31-44. doi: 10.1016/0301-0082(95)00019-r. Prog Neurobiol. 1995. PMID: 8570852 Review.
Cited by
- Cholinergic modulation of hippocampal network function.
Teles-Grilo Ruivo LM, Mellor JR. Teles-Grilo Ruivo LM, et al. Front Synaptic Neurosci. 2013 Jul 30;5:2. doi: 10.3389/fnsyn.2013.00002. eCollection 2013. Front Synaptic Neurosci. 2013. PMID: 23908628 Free PMC article. - Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses.
Cao G, Ko CP. Cao G, et al. J Neurosci. 2007 Jun 20;27(25):6712-22. doi: 10.1523/JNEUROSCI.1329-07.2007. J Neurosci. 2007. PMID: 17581958 Free PMC article. - beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons.
Liu Q, Kawai H, Berg DK. Liu Q, et al. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4734-9. doi: 10.1073/pnas.081553598. Epub 2001 Mar 27. Proc Natl Acad Sci U S A. 2001. PMID: 11274373 Free PMC article. - Antidromic-rectifying gap junctions amplify chemical transmission at functionally mixed electrical-chemical synapses.
Liu P, Chen B, Mailler R, Wang ZW. Liu P, et al. Nat Commun. 2017 Mar 20;8:14818. doi: 10.1038/ncomms14818. Nat Commun. 2017. PMID: 28317880 Free PMC article. - Neuromodulation of neurons and synapses.
Nadim F, Bucher D. Nadim F, et al. Curr Opin Neurobiol. 2014 Dec;29:48-56. doi: 10.1016/j.conb.2014.05.003. Epub 2014 Jun 5. Curr Opin Neurobiol. 2014. PMID: 24907657 Free PMC article. Review.
References
- Araujo DM, Lapchak PA, Collier B, Quirion R. Characterization of N-[3H]methylcarbamylcholine binding sites and effect of N-methylcarbamylcholine on acetylcholine release in rat brain. J Neurochem. 1988;51:292–299. - PubMed
- Arneric SP, Sullivan JP, Williams M. Neuronal nicotinic acetylcholine receptors. Novel targets for central nervous system therapeutics. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 95–110.
- Ascher P, Johnson JW. The NMDA receptor, its channel and its modulation by glycine. In: Watkins JC, Collingridge GL, editors. The NMDA receptor. Oxford UP; Oxford: 1989. pp. 109–121.
- Bertrand D, Changeux JP. Nicotinic receptor: an allosteric protein specialized for intercellular communication. Semin Neurosci. 1995;7:75–90.
- Boehm S, Huck S. Inhibition of N-type calcium channels: the only mechanism by which presynaptic α2-autoreceptors control sympathetic transmitter release. Eur J Neurosci. 1996;8:1924–1931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous