Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate - PubMed (original) (raw)
Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate
A Ritzhaupt et al. J Physiol. 1998.
Abstract
1. Oligonucleotide primers based on the human heart monocarboxylate transporter (MCT1) cDNA sequence were used to isolate a 544 bp cDNA product from human colonic RNA by reverse transcription-polymerase chain reaction (RT-PCR). The sequence of the RT-PCR product was identical to that of human heart MCT1. Northern blot analysis using the RT-PCR product indicated the presence of a single transcript of 3.3 kb in mRNA isolated from both human and pig colonic tissues. Western blot analysis using an antibody to human MCT1 identified a specific protein with an apparent molecular mass of 40 kDa in purified and well-characterized human and pig colonic lumenal membrane vesicles (LMV). 2. Properties of the colonic lumenal membrane L-lactate transporter were studied by the uptake of L-[U-14C]lactate into human and pig colonic LMV. L-Lactate uptake was stimulated in the presence of an outward-directed anion gradient at an extravesicular pH of 5.5. Transport of L-lactate into anion-loaded colonic LMV appeared to be via a proton-activated, anion exchange mechanism. 3. L-Lactate uptake was inhibited by pyruvate, butyrate, propionate and acetate, but not by Cl- and SO4(2-). The uptake of L-lactate was inhibited by phloretin, mercurials and alpha-cyano-4-hydroxycinnamic acid (4-CHC), but not by the stilbene anion exchange inhibitors, 4,4'-diisothiocyanostilbene-2, 2'-disulphonic acid (DIDS) and 4-acetamido-4'-isothiocyanostilbene-2, 2'-disulphonic acid (SITS). 4. The results indicate the presence of a MCT1 protein on the lumenal membrane of the colon that is involved in the transport of L-lactate as well as butyrate across the colonic lumenal membrane. Western blot analysis showed that the abundance of this protein decreases in lumenal membrane fractions isolated from colonic carcinomas compared with that detected in the normal healthy colonic tissue.
Figures
Figure 1. Detection of MCT1 transcript in pig and human colon by Northern blot analysis
Human and pig colonic RNA (5 μg lane−1, each) was fractionated on a 1% (w/v) denaturing agarose gel containing 0.66
m
formaldehyde, transferred to nylon membrane and probed with the radiolabelled human colonic RT-PCR product. Lane a, pig ileal RNA; lane b, pig colonic RNA; lane c, human ileal RNA; lane d, human colonic RNA.
Figure 2. Immunodetection of MCT1 protein in pig and human colonic LMV
LMV were dissolved in sample buffer containing SDS. Samples (20 μg (lane of protein)−1, each) were separated on an 8% polyacrylamide gel and electrotransferred to nitrocellulose membrane. Immunoblotting was carried out as described in Methods. The specificity of the immunoreaction was determined by comparing blots carried out under standard conditions (A) with those in which the MCT1 antibody had been pre-incubated with the immunizing peptide antigen (B). Lane a, pig colonic homogenate; lane b, pig colonic LMV; lane c, human colonic homogenate; lane d, human colonic LMV; lane e, rat erythrocyte ghosts.
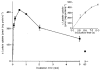
Figure 3. Time course of l-lactate uptake into pig colonic LMV
LMV were preloaded with standard loading buffer (100 m
m
mannitol, 100 m
m
sodium
l
-lactate, 20 m
m
Hepes-Tris pH 7.5 and 0.1 m
m
MgSO4). LMV (100 μg of protein per assay) were incubated in the standard uptake media containing 100 m
m
mannitol, 100 m
m
sodium gluconate, 20 m
m
Mes-Tris pH 5.5, 0.1 m
m
MgSO4, and 0.1 m
m
L-[U-14C]lactate. Uptake was measured at 37 °C as described. Values are presented as means ±
s.e.m.
for three experiments. The inset shows the linear phase of
l
-lactate uptake.
Figure 4. Saturability of l-lactate transport into pig colonic LMV
Initial rates of
l
-lactate transport with increasing lactate concentrations over the range of 1–40 m
m
were determined. LMV were loaded with 100 m
m
mannitol, 100 m
m
NaHCO3, 20 m
m
Hepes-Tris pH 7.5, and 0.1 m
m
MgSO4. LMV (100 μg protein per assay) were incubated in media containing 100 m
m
mannitol, 0.1 m
m
MgSO4, 20 m
m
Mes-Tris pH 5.5 and varying concentrations of sodium gluconate, sodium
l
-lactate (to maintain a constant media osmolarity and sodium concentration) and tracer amounts of L-[U-14C]lactate. Uptake was measured at 37 °C for 5 s. The data are presented as a Michaelis-Menten curve and (inset) as Wolf-Hanes plot of substrate concentration (S) against S/V, where V is the velocity, (_r_2 = 0.96). Values are presented as means ±
s.e.m.
for three experiments.
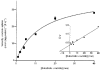
Figure 5. Inhibition of l-lactate transport by structural analogues
LMV isolated from pig ( ) and human (
) and human ( ) colon were preloaded with 100 m
) colon were preloaded with 100 m
m
mannitol, 100 m
m
sodium
l
-lactate, 20 m
m
Hepes-Tris pH 7.5 and 0.1 m
m
MgSO4. LMV (100 μg of protein per assay) were incubated in medium containing 100 m
m
mannitol, 80 m
m
sodium gluconate (60 m
m
sodium gluconate when Na2SO4 and sodium succinate were present in the media and 95 m
m
sodium gluconate when 4-CHC was present in the media), 20 m
m
Mes-Tris pH 5.5, 0.1 m
m
MgSO4, 0.1 m
m
L-[U-14C]lactate and 20 m
m
of the following sodium salts: acetate, propionate, butyrate, pyruvate, succinate, Cl−, SO42−. 4-CHC was added at a concentration of 5 m
m
. Uptake was measured at 37 °C for 5 s. Values are presented as means ±
s.e.m.
for three experiments. * Statistically significant (P < 0.05) from control.
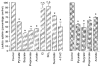
Figure 6. Immunodetection of MCT1 protein in healthy and diseased human colonic biopsies
Lumenal plasma membrane fractions were isolated from biopsy-size samples of several healthy, pre-cancerous and cancerous human colonic tissues. Protein fractions were dissolved in sample buffer containing SDS. Samples (20 μg (lane of protein)−1, each) were separated on an 8% polyacrylamide gel and electrotransferred to nitrocellulose membrane. Immunoblotting was carried out as described in Methods. Lane a, colon adenoma; lane b, colon adenoma; lane c, colon adenoma; lane d, rectal carcinoma; lane e, colon adenoma; lane f, colon carcinoma; lane g, healthy colon; lane h, healthy colon.
Similar articles
- Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon.
Mascolo N, Rajendran VM, Binder HJ. Mascolo N, et al. Gastroenterology. 1991 Aug;101(2):331-8. doi: 10.1016/0016-5085(91)90008-9. Gastroenterology. 1991. PMID: 2065907 - The characterization of butyrate transport across pig and human colonic luminal membrane.
Ritzhaupt A, Ellis A, Hosie KB, Shirazi-Beechey SP. Ritzhaupt A, et al. J Physiol. 1998 Mar 15;507 ( Pt 3)(Pt 3):819-30. doi: 10.1111/j.1469-7793.1998.819bs.x. J Physiol. 1998. PMID: 9508842 Free PMC article. - Mechanism of n-butyrate uptake in the human proximal colonic basolateral membranes.
Tyagi S, Venugopalakrishnan J, Ramaswamy K, Dudeja PK. Tyagi S, et al. Am J Physiol Gastrointest Liver Physiol. 2002 Apr;282(4):G676-82. doi: 10.1152/ajpgi.00173.2000. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11897627 - Lactate transporters (MCT proteins) in heart and skeletal muscles.
Bonen A. Bonen A. Med Sci Sports Exerc. 2000 Apr;32(4):778-89. doi: 10.1097/00005768-200004000-00010. Med Sci Sports Exerc. 2000. PMID: 10776897 Review. - Lactate transport in heart in relation to myocardial ischemia.
Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT. Halestrap AP, et al. Am J Cardiol. 1997 Aug 4;80(3A):17A-25A. doi: 10.1016/s0002-9149(97)00454-2. Am J Cardiol. 1997. PMID: 9293952 Review.
Cited by
- Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line.
Kekuda R, Manoharan P, Baseler W, Sundaram U. Kekuda R, et al. Dig Dis Sci. 2013 Mar;58(3):660-7. doi: 10.1007/s10620-012-2407-x. Epub 2013 Jan 24. Dig Dis Sci. 2013. PMID: 23344966 - The role of probiotics and prebiotics in inducing gut immunity.
Vieira AT, Teixeira MM, Martins FS. Vieira AT, et al. Front Immunol. 2013 Dec 12;4:445. doi: 10.3389/fimmu.2013.00445. Front Immunol. 2013. PMID: 24376446 Free PMC article. Review. - Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases.
Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Parada Venegas D, et al. Front Immunol. 2019 Mar 11;10:277. doi: 10.3389/fimmu.2019.00277. eCollection 2019. Front Immunol. 2019. PMID: 30915065 Free PMC article. Review. - Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease.
Lu Y, Zhang Y, Zhao X, Shang C, Xiang M, Li L, Cui X. Lu Y, et al. Front Cardiovasc Med. 2022 Aug 11;9:900381. doi: 10.3389/fcvm.2022.900381. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035928 Free PMC article. Review. - Lactose-Induced Chronic Diarrhea Results From Abnormal Luminal Microbial Fermentation and Disorder of Ion Transport in the Colon.
Xue H, Zhang M, Ma J, Chen T, Wang F, Tang X. Xue H, et al. Front Physiol. 2020 Jul 29;11:877. doi: 10.3389/fphys.2020.00877. eCollection 2020. Front Physiol. 2020. PMID: 32848839 Free PMC article.
References
- Alonso de la Torre SRA, Serrano MA, Medina JM. Carrier-mediated β-D-hydroxybutyrate transport in brush-border membrane vesicles from rat placenta. Pediatric Research. 1992;32:317–323. - PubMed
- Balkovetz DF, Leibach FH, Mahesh VB, Ganapathy V. A proton gradient is the driving force for uphill transport of lactate in human placental brush-border membrane vesicles. Journal of Biological Chemistry. 1988;263:13823–13830. - PubMed
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990;70:567–590. - PubMed
- Berry RD, Paraskeva C. Expression of a carcinoembryonic antigen by the adenoma and carcinoma derived epithelial cell lines: possible marker of tumour progression and modulation of expression by sodium butyrate. Carcinogenesis. 1988;9:447–450. - PubMed
- Bröer S, Raham B, Pellegri G, Martin J-L, Verleysdonk S, Hamprecht B, Magistetti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter I (MCT1) expressing Xenopus laevis oocytes. Journal of Biological Chemistry. 1997;272:30096–30102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources