Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes - PubMed (original) (raw)
Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes
J Pizarro-Cerdá et al. Infect Immun. 1998 Dec.
Abstract
Brucella abortus is an intracellular pathogen that replicates within a membrane-bounded compartment. In this study, we have examined the intracellular pathway of the virulent B. abortus strain 2308 (S2308) and the attenuated strain 19 (S19) in HeLa cells. At 10 min after inoculation, both bacterial strains are transiently detected in phagosomes characterized by the presence of early endosomal markers such as the early endosomal antigen 1. At approximately 1 h postinoculation, bacteria are located within a compartment positive for the lysosome-associated membrane proteins (LAMPs) and the endoplasmic reticulum (ER) marker sec61beta but negative for the mannose 6-phosphate receptors and cathepsin D. Interestingly, this compartment is also positive for the autophagosomal marker monodansylcadaverin, suggesting that S2308 and S19 are located in autophagic vacuoles. At 24 h after inoculation, attenuated S19 is degraded in lysosomes, while virulent S2308 multiplies within a LAMP- and cathepsin D-negative but sec61beta- and protein disulfide isomerase-positive compartment. Furthermore, treatment of infected cells with the pore-forming toxin aerolysin from Aeromonas hydrophila causes vacuolation of the bacterial replication compartment. These results are compatible with the hypothesis that pathogenic B. abortus exploits the autophagic machinery of HeLa cells to establish an intracellular niche favorable for its replication within the ER.
Figures
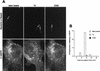
FIG. 1
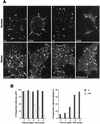
EEA1 is detected in _Brucella_-containing phagosomes. HeLa cells were inoculated with S2308 or S19 or were fed with latex beads for different times up to 20 min and then were processed for single (latex beads) or double indirect immunofluorescence (for incubation times longer than 20 min, cells were washed and further incubated with fresh cell culture medium containing gentamicin). (A) Distribution of EEA1 (lower panels) and latex beads, S19, and S2308 (upper panels) at 10 min after internalization. (B) Kinetics of acquisition of EEA1 by phagosomes. Internalized particles are labeled by EEA1 (arrows in panel A), with a maximal acquisition of EEA1 at 10 min postinoculation (B). In panel B, data are averages from two different experiments. The percentage of phagosomes containing EEA1 was calculated as described in Materials and Methods. Bar, 5 μm.
FIG. 2
_Brucella_-containing phagosomes avoid interaction with CI-M6PR-positive compartments. HeLa cells were fed with latex beads or inoculated with S2308 or S19 for different times and processed for immunofluorescence as described in the legend to Fig. 1. (A) Distribution of CI-M6PR (lower panels) and latex beads, S19, and S2308 (upper panels) at 30 min after inoculation. (B) Kinetics of acquisition of CI-M6PR by phagosomes. Note that only latex beads are decorated by anti-CI-M6PR antibodies (arrows in panel A). At 30 min postinoculation, some S19 bacteria are found in late phagosomes, while maximal acquisition of CI-M6PR is observed at 30 min in latex bead-containing phagosomes (B). In panel B, data are averages from two different experiments. The percentage of phagosomes containing CI-M6PR was calculated as described in Materials and Methods. Bar, 5 μm.
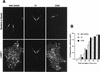
FIG. 3
LAMP1 distributes in _Brucella_-containing phagosomes. HeLa cells were fed with latex beads or inoculated with S2308 or S19 for different times and were processed for immunofluorescence as described in the legend to Fig. 1. (A) Distribution of LAMP1 (lower panels) and latex beads, S19, and S2308 (upper panels) at 1 h after inoculation. (B) Kinetics of acquisition of LAMP1 by phagosomes. LAMP1 labeling is detected in both Brucella- and latex bead-containing phagosomes (arrows in panel A), with >80% LAMP1-positive _Brucella-_containing phagosomes at 90 min of internalization (B). In panel B, data are averages from two different experiments. The percentage of phagosomes containing LAMP1 was calculated as described in Materials and Methods. Bar, 5 μm.
FIG. 4
Cathespin D is not expressed in _Brucella_-containing phagosomes. HeLa cells were fed with latex beads or inoculated with S2308 or S19 for different times and processed for immunofluorescence as described in the legend to Fig. 1. (A) Distribution of cathepsin D (lower panels) and latex beads, S19, and S2308 (upper panels) at 1 h after inoculation. (B) Kinetics of acquisition of cathepsin D by phagosomes. While phagosomes containing latex beads are abundantly labeled by cathepsin D (arrows in panel A) the lysosomal marker is absent from _Brucella-_containing phagosomes (arrows in panel A). Only a few phagosomes containing S2308 (<1%) or S19 (<5%) colocalize with cathepsin D at 2 h postinoculation (B). In panel B, data are averages from two different experiments. The percentage of phagosomes containing cathepsin D was calculated as described in Materials and Methods. Bar, 5 μm.
FIG. 5
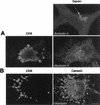
_Brucella-_containing phagosomes express the ER marker sec61β. HeLa cells were inoculated with S2308 or S19 for different times and processed for immunofluorescence as described in the legend to Fig. 1. (A) Distribution of sec61β (lower panels) at 1 h postinoculation with the corresponding bacteria (upper panels). (B) Kinetics of acquisition of sec61β on phagosomes. Both S2308 and S19 are found in compartments labeled by sec61β (arrows in panel A). sec61β is incorporated in _Brucella-_containing phagosomes (B) with kinetics similar to that of LAMP1 (Fig. 3). In panel B, data are averages from two different experiments. The percentage of phagosomes containing sec61β was calculated as described in Materials and Methods. Bar, 5 μm.
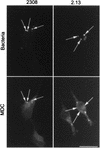
FIG. 6
MDC colocalizes with S2308-containing phagosomes. HeLa cells were inoculated for 1 h with S2308 or S2.13, washed, and further incubated for 30 min with cell culture medium depleted of fetal calf serum and glutamine and supplemented with gentamicin. Monolayers were then incubated for 30 min with MDC (0.05 mM), washed, and processed for immunofluorescence. Only vesicles containing S2308 (arrows) are abundantly labeled with MDC. Bar, 10 μm.
FIG. 7
S2308 multiplies in LAMP1- and cathepsin D-negative compartments. HeLa cells were inoculated for 1 h with S2308 and S19, washed, and further incubated with cell culture medium with gentamicin. At different times postinoculation, monolayers were fixed and processed for double indirect immunofluorescence. (A) Distribution of LAMP1 (two lower left panels) and cathepsin D (two lower right panels) with the indicated bacteria (upper panels) at 24 h after inoculation. (B) Kinetics of LAMP1 (left panel) and cathepsin D (right panel) acquisition in phagosomes. S19 is degraded after 24 h postinoculation (A), and both intact bacteria (arrows) and degradation products (arrowheads) colocalize with the lysosomal markers LAMP1 and cathepsin D. Moreover, S19 gradually acquires cathepsin D (B, right panel). S2308 is able to multiply in a LAMP1 and cathepsin D-negative compartment (A). LAMP is excluded from the S2308-containing phagosomes from 8 h after inoculation onwards (B, left panel). In panel B, data are averages from two different experiments. The percentages of phagosomes containing LAMP1 or cathepsin D were calculated as described in Materials and Methods. Bar, 5 μm.
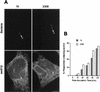
FIG. 8
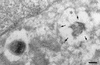
Rare S2308 organisms are detected in LAMP1-positive compartments at 48 h after inoculation. HeLa cell monolayers were infected for 24 h with S2308, washed, and further incubated in cell culture medium with gentamicin for an additional 24 h. Monolayers were then fixed and processed for frozen sectioning, immunolabeling, and electron microscopy analysis. Sections were labeled for LAMP1 followed by 10-nm-gold-conjugated antibody. The micrograph shows two bacteria. The bacterium on the right shows signs of degradation, and its vacuole is positive for LAMP1 (arrows). In contrast, the healthy bacterium on the left is devoided of LAMP1. Bar, 200 nm.
FIG. 9
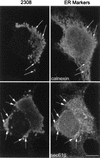
S2308 multiplies in the ER. HeLa cells were infected with S2308 for 1 h, washed, and further incubated with cell culture medium supplemented with gentamicin. At 24 h after inoculation, cells were fixed and processed for double immunofluorescence. Multiplying bacteria are located in a perinuclear compartment (arrows) that matches the distribution of sec61β (lower panels) and calnexin (upper panels). Bar, 5 μm.
FIG. 10
The S2308 replication compartment retains functional features of the ER. Cells were infected for 1 h with S2308, washed, and further incubated with cell culture medium in the presence of gentamicin. At 24 h postinoculation, cells were incubated for 30 min with brefeldin A (10 μg/ml) (A) or for 55 min with proaerolysin (0.38 nM) (B). Monolayers were then fixed and processed for double indirect immunofluorescence. (A) The upper right panel shows the distribution of the Golgi compartment (as detected by an antigiantin antibody [arrow]) in a nontreated cell. The lower panels show the distributions of giantin (right panel) and S2308 (left panel) in the same brefeldin A-treated cell. The Golgi is redistributed in an intracellular location that matches the distribution of the bacteria (as defined by the region delimited by the dotted line). (B) Distributions of calnexin (right panel) and S2308 (left panel) in a proaerolysin-treated cell. The bacterial replication compartment is disorganized and colocalizes with the vacuolated ER (arrows). Bar, 5 μm.
FIG. 11
S2308 is located in a PDI-positive compartment. HeLa cell monolayers were infected for 24 h with S2308, washed, and further incubated in cell culture medium with gentacimin for additional 24 h. Monolayers were then fixed and processed for frozen sectioning, immunolabeling, and electron microscopy analysis. Sections were labeled for PDI followed by 10-nm-gold-conjugated antibody. Arrows indicate the specific labeling for PDI associated with the bacterium-containing vacuoles. (A) Bar, 200 nm. (B) Bar, 100 nm.
FIG. 12
Proposed model of the intracellular traffic of B. abortus in HeLa cells. Both virulent strain S2308 and attenuated strain S19 are found within 10 min after invasion in an early compartment positive for EEA1 that is able to fuse with autophagosomes originating from the ER and enriched by LAMP molecules possibly derived from the trans-Golgi network. S19 is then unable to inhibit the maturation of its autophagosome, which fuses with lysosomes and causes its degradation. In contrast, S2308 diverts the maturation pathway of autophagosomes and uses a retrograde transport system to access the ER, where massive replication occurs.
Similar articles
- Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes.
Bellaire BH, Roop RM 2nd, Cardelli JA. Bellaire BH, et al. Infect Immun. 2005 Jun;73(6):3702-13. doi: 10.1128/IAI.73.6.3702-3713.2005. Infect Immun. 2005. PMID: 15908400 Free PMC article. - Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments.
Pizarro-Cerdá J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Pizarro-Cerdá J, et al. Infect Immun. 1998 May;66(5):2387-92. doi: 10.1128/IAI.66.5.2387-2392.1998. Infect Immun. 1998. PMID: 9573138 Free PMC article. - Intracellular trafficking of Brucella abortus in J774 macrophages.
Arenas GN, Staskevich AS, Aballay A, Mayorga LS. Arenas GN, et al. Infect Immun. 2000 Jul;68(7):4255-63. doi: 10.1128/IAI.68.7.4255-4263.2000. Infect Immun. 2000. PMID: 10858243 Free PMC article. - Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells.
Pizarro-Cerdá J, Moreno E, Gorvel JP. Pizarro-Cerdá J, et al. Microbes Infect. 2000 Jun;2(7):829-35. doi: 10.1016/s1286-4579(00)90368-x. Microbes Infect. 2000. PMID: 10955964 Review. - Bacterial interactions with the autophagic pathway.
Dorn BR, Dunn WA Jr, Progulske-Fox A. Dorn BR, et al. Cell Microbiol. 2002 Jan;4(1):1-10. doi: 10.1046/j.1462-5822.2002.00164.x. Cell Microbiol. 2002. PMID: 11856168 Review.
Cited by
- Autophagy favors Brucella melitensis survival in infected macrophages.
Guo F, Zhang H, Chen C, Hu S, Wang Y, Qiao J, Ren Y, Zhang K, Wang Y, Du G. Guo F, et al. Cell Mol Biol Lett. 2012 Jun;17(2):249-57. doi: 10.2478/s11658-012-0009-4. Epub 2012 Feb 24. Cell Mol Biol Lett. 2012. PMID: 22367856 Free PMC article. - Bacterial Type IV secretion systems: versatile virulence machines.
Voth DE, Broederdorf LJ, Graham JG. Voth DE, et al. Future Microbiol. 2012 Feb;7(2):241-57. doi: 10.2217/fmb.11.150. Future Microbiol. 2012. PMID: 22324993 Free PMC article. Review. - Immune Response to Mucosal Brucella Infection.
López-Santiago R, Sánchez-Argáez AB, De Alba-Núñez LG, Baltierra-Uribe SL, Moreno-Lafont MC. López-Santiago R, et al. Front Immunol. 2019 Aug 20;10:1759. doi: 10.3389/fimmu.2019.01759. eCollection 2019. Front Immunol. 2019. PMID: 31481953 Free PMC article. Review. - The Brucella abortus phosphoglycerate kinase mutant is highly attenuated and induces protection superior to that of vaccine strain 19 in immunocompromised and immunocompetent mice.
Trant CG, Lacerda TL, Carvalho NB, Azevedo V, Rosinha GM, Salcedo SP, Gorvel JP, Oliveira SC. Trant CG, et al. Infect Immun. 2010 May;78(5):2283-91. doi: 10.1128/IAI.01433-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194591 Free PMC article. - Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro.
Eze MO, Yuan L, Crawford RM, Paranavitana CM, Hadfield TL, Bhattacharjee AK, Warren RL, Hoover DL. Eze MO, et al. Infect Immun. 2000 Jan;68(1):257-63. doi: 10.1128/IAI.68.1.257-263.2000. Infect Immun. 2000. PMID: 10603396 Free PMC article.
References
- Andrews N W, Whitlow M B. Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol Biochem Parasitol. 1989;33:249–256. - PubMed
- Aplin A, Jasionowski T, Tuttle D L, Lenk S E, Dunn W A. Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J Cell Physiol. 1992;152:458–466. - PubMed
- Baldwin C L, Winter A J. Macrophages and Brucella. Immunol Semin. 1994;60:363–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources