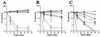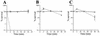The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum - PubMed (original) (raw)
The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum
C M Kahler et al. Infect Immun. 1998 Dec.
Abstract
The molecular basis for the resistance of serogroup B Neisseria meningitidis to the bactericidal activity of normal human sera (NHS) was examined with a NHS-resistant, invasive serogroup B meningococcal isolate and genetically and structurally defined capsule-, lipooligosaccharide (LOS)-, and sialylation-altered mutants of the wild-type strain. Expression of the (alpha2-->8)-linked polysialic acid serogroup B capsule was essential for meningococcal resistance to NHS. The very NHS-sensitive phenotype of acapsular mutants (99.9 to 100% killed in 10, 25, and 50% NHS) was not rescued by complete LOS sialylation or changes in LOS structure. However, expression of the capsule was necessary but not sufficient for a fully NHS-resistant phenotype. In an encapsulated background, loss of LOS sialylation by interrupting the alpha2,3 sialyltransferase gene, lst, increased sensitivity to 50% NHS. In contrast, replacement of the lacto-N-neotetraose alpha-chain (Galbeta1-4GlcNAcbeta1-3Galbeta1-4Glc) with glucose extensions (GlcN) in a galE mutant resulted in a strain resistant to killing by 50% NHS at all time points. Encapsulated meningococci expressing a Hep2(GlcNAc)-->KDO2-->lipid A LOS without an alpha-chain demonstrated enhanced sensitivity to 50% NHS (98% killed at 30 min) mediated through the antibody-dependent classical complement pathway. Encapsulated LOS mutants expressing truncated Hep2-->KDO2-->lipid A and KDO2-->lipid A structures were also sensitive to 50% NHS (98 to 100% killed at 30 min) but, unlike the wild-type strain and mutants with larger oligosaccharide structures, they were killed by hypogammaglobulinemic sera. These data indicate that encapsulation is essential but that the LOS structure contributes to the ability of serogroup B N. meningitidis to resist the bactericidal activity of NHS.
Figures
FIG. 1
Predominant LOS structure of the serogroup B N. meningitidis strain NMB and LOS structures expressed by a series of genetically defined NMB mutants. (A) The predominant LOS of parental strain NMB, (NANAα2→3)Galβ1→4GlcNAcβ1→3Galβ1→4Glc→Hep2(GlcNAc,Glc)PEA→KDO2, contains a terminal lacto-_N_-neotetraose (Galβ1→4GlcNAcβ1→3Galβ1→4Glc) in the α-chain, with a portion of the molecules containing a terminally linked sialic acid attached to the 0-3 lacto-_N_-neotetraose galactosyl residue and two heptoses attached through two KDOs to lipid A (41). (B) In contrast to the LOS of the wild-type strain, NMB, the LOS lacto-_N_-neotetraose of the sialic acid biosynthesis pathway mutant, M7 (_synA_− [_siaA_−]), and the α2→3 sialyltransferase mutant, Lst (_lst_−), lacks the terminally linked sialic acid. (C) In the UDP-Glc4-epimerase galE mutant, SS3, mass spectrometric analysis of O_-deacylated LOS revealed the presence of multiple species, with the predominant LOS species in this mutant strain formed by the GlcN→Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2 (32). (D) The LOS from HepIβ1-4 glucosyl transferase mutant NMB_lgtF::aphA-3 (nonpolar lgtF mutant) had a GlcNAc/Hep ratio of 1:2.125 with no other hexoses detected, indicating that the predominant structure was Hep2(GlcNAc)KDO2→lipid A (29). The phosphoglucomutase pgm mutant, R6, has an identical structure (69). (E) Glycosyl composition and linkage analysis of _rfaK_− (α1,2 _N_-acetylglucosamine transferase mutant) LOS before and after de-O-acetylation showed that the oligosaccharide of this LOS consists of only Hep and KDO and that a PEA group is attached to position 3 of HepII. In addition, no glucose is present in the _rfaK_− LOS structure. Fast atom bombardment-mass spectrometry of the de-_O_-acylated LOS and one-dimensional proton NMR analyses of the oligosaccharide gave results that were consistent with the structure (29). (F) Electrospray-mass spectrometry of the de-_O_-acylated mutant 469 (_orfA_−) LOS preparation indicated that it contained one major molecular species, KDO2→lipid A.
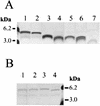
FIG. 2
LOS structures of N. meningitidis NMB and genetically defined LOS mutants separated by Tricine SDS-PAGE and visualized by silver staining (30). (A) The NMB parental LOS structure (lane 1) is compared to the truncated LOS from NMB_lst_::Ω (lane 2), SS3 (_galE_−) (lane 3), R6 (_pgm_−) (lane 4), CMK2 (_lgtF_−) (lane 5), CMK1 (_rfaK_−) (lane 6), and 469 (_orfA_−) (lane 7). (B) The parent strain, NMB (lane 1), expresses LOS which is partially sialylated under normal growth conditions and which separates as two bands in Tricine SDS-PAGE. Mutant 43 (_synD_−) (lane 2) expresses LOS which is almost completely sialylated, since little further sialylation can be achieved when the strain is grown in the presence of 50 μg of CMP-NANA/ml (lane 3). In comparison, mutant M7 (_synA_−) cannot synthesize sialic acid and expresses only nonsialylated LOS (lane 4). Molecular mass standards (Boehringer Mannheim) are also shown.
FIG. 3
Survival in 10 (A), 25 (B), and 50% (C) NHS of the wild-type parental meningococcal strain, NMB (□); sialic acid or capsule pathway mutants expressing different sialylation phenotypes (_synA_− [■], synD_− [○], and NMB_lst::Ω [•]; or mutants expressing truncated LOS structures (SS3 [_galE_−] [◊], R6 [_pgm_−], [⧫], CMK2 [_lgtF_−], [∗], CMK1 [_rfaK_−] [▵], and 469 [_orfA_−] [▴]). Error bars indicate standard error of the means.
FIG. 4
Resistances to NHS of the parental gonococcal strain, FA19 (□), and the LOS mutants containing the pgm mutation (FA19/R6) (◊) and the orfA mutation (FA19/469) (–––) are shown at 10 (A), 25 (B), and 50% (C) NHS. Error bars indicate standard error of the means.
Similar articles
- Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis.
Vogel U, Weinberger A, Frank R, Müller A, Köhl J, Atkinson JP, Frosch M. Vogel U, et al. Infect Immun. 1997 Oct;65(10):4022-9. doi: 10.1128/iai.65.10.4022-4029.1997. Infect Immun. 1997. PMID: 9317002 Free PMC article. - Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the alpha1,2 N-acetylglucosamine transferase (RfaK).
Kahler CM, Carlson RW, Rahman MM, Martin LE, Stephens DS. Kahler CM, et al. J Bacteriol. 1996 Mar;178(5):1265-73. doi: 10.1128/jb.178.5.1265-1273.1996. J Bacteriol. 1996. PMID: 8631701 Free PMC article. - The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis.
Ram S, Mackinnon FG, Gulati S, McQuillen DP, Vogel U, Frosch M, Elkins C, Guttormsen HK, Wetzler LM, Oppermann M, Pangburn MK, Rice PA. Ram S, et al. Mol Immunol. 1999 Sep-Oct;36(13-14):915-28. doi: 10.1016/s0161-5890(99)00114-5. Mol Immunol. 1999. PMID: 10698346 Review.
Cited by
- Discovery and characterization of de novo sialic acid biosynthesis in the phylum Fusobacterium.
Lewis AL, Robinson LS, Agarwal K, Lewis WG. Lewis AL, et al. Glycobiology. 2016 Oct;26(10):1107-1119. doi: 10.1093/glycob/cww068. Epub 2016 Sep 9. Glycobiology. 2016. PMID: 27613803 Free PMC article. - Modeling Neisseria meningitidis metabolism: from genome to metabolic fluxes.
Baart GJ, Zomer B, de Haan A, van der Pol LA, Beuvery EC, Tramper J, Martens DE. Baart GJ, et al. Genome Biol. 2007;8(7):R136. doi: 10.1186/gb-2007-8-7-r136. Genome Biol. 2007. PMID: 17617894 Free PMC article. - Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability-increasing protein are influenced by the capsulation and lipooligosaccharide structure of Neisseria meningitidis.
Dixon GL, Heyderman RS, Kotovicz K, Jack DL, Andersen SR, Vogel U, Frosch M, Klein N. Dixon GL, et al. Infect Immun. 1999 Nov;67(11):5626-33. doi: 10.1128/IAI.67.11.5626-5633.1999. Infect Immun. 1999. PMID: 10531209 Free PMC article. - Polymorphisms in pilin glycosylation Locus of Neisseria meningitidis expressing class II pili.
Kahler CM, Martin LE, Tzeng YL, Miller YK, Sharkey K, Stephens DS, Davies JK. Kahler CM, et al. Infect Immun. 2001 Jun;69(6):3597-604. doi: 10.1128/IAI.69.6.3597-3604.2001. Infect Immun. 2001. PMID: 11349019 Free PMC article. - Attachment and invasion of Neisseria meningitidis to host cells is related to surface hydrophobicity, bacterial cell size and capsule.
Bartley SN, Tzeng YL, Heel K, Lee CW, Mowlaboccus S, Seemann T, Lu W, Lin YH, Ryan CS, Peacock C, Stephens DS, Davies JK, Kahler CM. Bartley SN, et al. PLoS One. 2013;8(2):e55798. doi: 10.1371/journal.pone.0055798. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405216 Free PMC article.
References
- Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. - PubMed
- Andersen S R, Bjune G, Høiby E A, Michaelsen T E, Aase A, Rye U, Jantzen E. Outer membrane vesicle vaccines made from short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis: effect of the carbohydrate chain length on the immune response. Microb Pathog. 1997;23:139–155. - PubMed
- Andersen S R, Kolberg J, Høiby E A, Namork E, Caugant D A, Frøholm L O, Jantzen E, Bjune G. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: possible consequences for vaccine development. Microb Pathog. 1997;23:139–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources