Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice - PubMed (original) (raw)
Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice
M Barton et al. Proc Natl Acad Sci U S A. 1998.
Abstract
This study investigated whether endothelin-1 (ET-1), a potent vasoconstrictor, which also stimulates cell proliferation, contributes to endothelial dysfunction and atherosclerosis. Apolipoprotein E (apoE)-deficient mice and C57BL/6 control mice were treated with a Western-type diet to accelerate atherosclerosis with or without ETA receptor antagonist LU135252 (50 mg/kg/d) for 30 wk. Systolic blood pressure, plasma lipid profile, and plasma nitrate levels were determined. In the aorta, NO-mediated endothelium-dependent relaxation, atheroma formation, ET receptor-binding capacity, and vascular ET-1 protein content were assessed. In apoE-deficient but not C57BL/6 mice, severe atherosclerosis developed within 30 wk. Aortic ET-1 protein content (P < 0.0001) and binding capacity for ETA receptors was increased as compared with C57BL/6 mice. In contrast, NO-mediated, endothelium-dependent relaxation to acetylcholine (56 +/- 3 vs. 99 +/- 2%, P < 0.0001) and plasma nitrate were reduced (57.9 +/- 4 vs. 93 +/- 10 micromol/liter, P < 0.01). Treatment with the ETA receptor antagonist LU135252 for 30 wk had no effect on the lipid profile or systolic blood pressure in apoE-deficient mice, but increased NO-mediated endothelium-dependent relaxation (from 56 +/- 3 to 93 +/- 2%, P < 0.0001 vs. untreated) as well as circulating nitrate levels (from 57.9 +/- 4 to 80 +/- 8.3 micromol/liter, P < 0.05). Chronic ETA receptor blockade reduced elevated tissue ET-1 levels comparable with those found in C57BL/6 mice and inhibited atherosclerosis in the aorta by 31% without affecting plaque morphology or ET receptor-binding capacity. Thus, chronic ETA receptor blockade normalizes NO-mediated endothelial dysfunction and reduces atheroma formation independent of plasma cholesterol and blood pressure in a mouse model of human atherosclerosis. ETA receptor blockade may have therapeutic potential in patients with atherosclerosis.
Figures
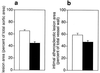
Figure 1
Quantification of atherosclerosis in the aorta of control apoE-deficient mice (white) and mice treated with LU135252 for 30 wk (black). Intimal area of the aortic surface area covered with atheromatous plaques (a) and lesion size in histologic sections of the thoracic aorta (b) were reduced by chronic ETA receptor blockade. No macroscopic or microscopic lesions were observed in C57BL/6 mice (unpublished observation and ref. 34). P < 0.001 (a) and P < 0.05 (b) for control vs. LU135252.
Figure 2
Photomicrographs of atherosclerotic lesion in the aortic arch of apoE-deficient mice. Advanced intimal lesion at the root of the carotid artery in an untreated apoE-deficient mouse exhibiting necrosis, calcification, and cholesterol crystals (a). Lesion at the same location in an apoE-deficient mouse after 30 wk of ETA receptor blockade (b). Cellular composition and morphology of the plaques was similar to that seen in untreated animals. (Elastica van Giesson stain, original magnification X60).
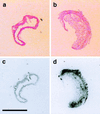
Figure 3
Photomicrographs and autoradiographs of the aorta from C57BL/6 (Left) and apoE-deficient mice (Right). Representative histologic sections from the aorta of a C57BL/6 control mouse (a) and an atherosclerotic apoE-deficient mouse (b). Note the large plaque occluding the lumen in the aorta of the apoE-deficient mouse (b). In these animals, endothelin binding by using [125I]ET-1 was markedly increased as compared with C57BL/6 mice (c) and was localized both in the vascular wall and in the atheromatous plaque (d). (Hematoxilin/eosin stain, original magnification X10. Bar: 1,000 μm).
Figure 4
Effects of chronic ETA receptor blockade (black) on aortic ET-1 content and vascular function in isolated aortic rings of apoE-deficient mice compared with untreated animals (white). Endothelin blockade reduced aortic ET-1 protein content comparable to levels of C57BL/6 mice (a). NO-mediated endothelium-dependent relaxation to acetylcholine was normalized by treatment with the ETA antagonist LU135252 (b) and inversely correlated with vascular ET-1 protein content. Sensitivity to exogenous NO as assessed by relaxations to sodium nitroprusside was slightly enhanced (c). Analyses were performed on n = 8–10 (a) and n = 8 (b and c) from each group. P < 0.0001 control vs. untreated.
Similar articles
- ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension.
Barton M, d'Uscio LV, Shaw S, Meyer P, Moreau P, Lüscher TF. Barton M, et al. Hypertension. 1998 Jan;31(1 Pt 2):499-504. doi: 10.1161/01.hyp.31.1.499. Hypertension. 1998. PMID: 9453352 - Chronic ET(A) receptor blockade prevents endothelial dysfunction of small arteries in apolipoprotein E-deficient mice.
d'Uscio LV, Barton M, Shaw S, Lüscher TF. d'Uscio LV, et al. Cardiovasc Res. 2002 Feb 1;53(2):487-95. doi: 10.1016/s0008-6363(01)00469-2. Cardiovasc Res. 2002. PMID: 11827700 - Endothelin 1 type a receptor antagonism prevents vascular dysfunction and hypertension induced by 11beta-hydroxysteroid dehydrogenase inhibition: role of nitric oxide.
Ruschitzka F, Quaschning T, Noll G, deGottardi A, Rossier MF, Enseleit F, Hürlimann D, Lüscher TF, Shaw SG. Ruschitzka F, et al. Circulation. 2001 Jun 26;103(25):3129-35. doi: 10.1161/01.cir.103.25.3129. Circulation. 2001. PMID: 11425780 - Endothelial dysfunction and atherosclerosis: endothelin receptor antagonists as novel therapeutics.
Barton M. Barton M. Curr Hypertens Rep. 2000 Feb;2(1):84-91. doi: 10.1007/s11906-000-0064-5. Curr Hypertens Rep. 2000. PMID: 10981133 Review. - Endothelin in atherosclerosis: importance of risk factors and therapeutic implications.
d'Uscio LV, Barton M, Shaw S, Lüscher TF. d'Uscio LV, et al. J Cardiovasc Pharmacol. 2000;35(4 Suppl 2):S55-59. doi: 10.1097/00005344-200000002-00013. J Cardiovasc Pharmacol. 2000. PMID: 10976783 Review.
Cited by
- The Role of Polymorphism in the Endothelial Homeostasis and Vitamin D Metabolism Genes in the Severity of Coronary Artery Disease.
Ponasenko A, Sinitskaya A, Sinitsky M, Khutornaya M, Barbarash O. Ponasenko A, et al. Biomedicines. 2023 Aug 25;11(9):2382. doi: 10.3390/biomedicines11092382. Biomedicines. 2023. PMID: 37760823 Free PMC article. - Epigenetically altered macrophages promote development of diabetes-associated atherosclerosis.
Huang D, Gao W, Zhong X, Wu H, Zhou Y, Ma Y, Qian J, Ge J. Huang D, et al. Front Immunol. 2023 May 5;14:1196704. doi: 10.3389/fimmu.2023.1196704. eCollection 2023. Front Immunol. 2023. PMID: 37215106 Free PMC article. - Endothelin Receptor Antagonists in Kidney Disease.
Martínez-Díaz I, Martos N, Llorens-Cebrià C, Álvarez FJ, Bedard PW, Vergara A, Jacobs-Cachá C, Soler MJ. Martínez-Díaz I, et al. Int J Mol Sci. 2023 Feb 8;24(4):3427. doi: 10.3390/ijms24043427. Int J Mol Sci. 2023. PMID: 36834836 Free PMC article. Review. - Endothelin receptor antagonists in kidney protection for diabetic kidney disease and beyond?
Chung EYM, Badve SV, Heerspink HJL, Wong MG. Chung EYM, et al. Nephrology (Carlton). 2023 Feb;28(2):97-108. doi: 10.1111/nep.14130. Epub 2022 Nov 15. Nephrology (Carlton). 2023. PMID: 36350038 Free PMC article. Review.
References
- Ross R. Nature (London) 1993;362:801–809. - PubMed
- Lüscher T F, Tanner F C, Tschudi M R, Noll G. Annu Rev Med. 1993;44:395–418. - PubMed
- Flavahan N A. Circulation. 1992;85:1927–1938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous