Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase - PubMed (original) (raw)
Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase
E E Sander et al. J Cell Biol. 1998.
Abstract
We previously demonstrated that both Tiam1, an activator of Rac, and constitutively active V12Rac promote E-cadherin-mediated cell-cell adhesion in epithelial Madin Darby canine kidney (MDCK) cells. Moreover, Tiam1 and V12Rac inhibit invasion of Ras-transformed, fibroblastoid MDCK-f3 cells by restoring E-cadherin-mediated cell-cell adhesion. Here we show that the Tiam1/Rac-induced cellular response is dependent on the cell substrate. On fibronectin and laminin 1, Tiam1/Rac signaling inhibits migration of MDCK-f3 cells by restoring E-cadherin-mediated cell- cell adhesion. On different collagens, however, expression of Tiam1 and V12Rac promotes motile behavior, under conditions that prevent formation of E-cadherin adhesions. In nonmotile cells, Tiam1 is present in adherens junctions, whereas Tiam1 localizes to lamellae of migrating cells. The level of Rac activation by Tiam1, as determined by binding to a glutathione-S-transferase- PAK protein, is similar on fibronectin or collagen I, suggesting that rather the localization of the Tiam1/Rac signaling complex determines the substrate-dependent cellular responses. Rac activation by Tiam1 requires PI3-kinase activity. Moreover, Tiam1- but not V12Rac-induced migration as well as E-cadherin-mediated cell- cell adhesion are dependent on PI3-kinase, indicating that PI3-kinase acts upstream of Tiam1 and Rac.
Figures
Figure 2
Morphological effects of matrix composition on C1199Tiam1-expressing MDCK-f3 cells. Small clusters of C1199Tiam1-expressing MDCK-f3 cells were seeded in the presence of HGF on (A) fibronectin (10 μg/ml), (B) a mixed matrix of fibronectin (10 μg/ml) and collagen I (0.1 μg/ml), (C) fibronectin (for 3 h), followed by addition of 10 μg/ml soluble collagen I, or (D) collagen IV (10 μg/ml). Phase–contrast photographs were taken 5 h after seeding. Bar, 25 μm.
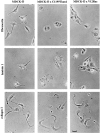
Figure 1
Reversion of the fibroblastoid phenotype of Ras-transformed MDCK-f3 cells to an epithelioid phenotype by C1199Tiam1 or V12 Rac is dependent on the cell substrate. Control MDCK-f3 cells and C1199Tiam1- or V12Rac-expressing MDCK-f3 cells were seeded in small clusters of 2–4 cells on fibronectin, laminin 1, or collagen I in the presence of HGF. Phase– contrast photographs were taken 10 h after seeding. Bar, 25 μm.
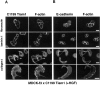
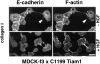
Figure 3
Subcellular localization of C1199Tiam1 expressed in MDCK-f3 cells is dependent on cell–matrix interactions. (A–C) Trypsinized C1199Tiam1-expressing MDCK-f3 cells were seeded as small clusters of 2–4 cells on glass coverslips coated with different matrices (10 μg/ml) in the presence of HGF, and stained for Tiam1 and F-actin (A), E-cadherin and F-actin (B), or Tiam1 and E-cadherin (C). The cells established E-cadherin–mediated adhesions on fibronectin and laminin1, but appeared fibroblastoid on collagen I. Note that C1199Tiam1 colocalizes with E-cadherin in adherens junctions. Immunofluorescence and confocal laser scanning microscopy was performed 10 h after seeding. Bar, 25 μm.
Figure 3
Subcellular localization of C1199Tiam1 expressed in MDCK-f3 cells is dependent on cell–matrix interactions. (A–C) Trypsinized C1199Tiam1-expressing MDCK-f3 cells were seeded as small clusters of 2–4 cells on glass coverslips coated with different matrices (10 μg/ml) in the presence of HGF, and stained for Tiam1 and F-actin (A), E-cadherin and F-actin (B), or Tiam1 and E-cadherin (C). The cells established E-cadherin–mediated adhesions on fibronectin and laminin1, but appeared fibroblastoid on collagen I. Note that C1199Tiam1 colocalizes with E-cadherin in adherens junctions. Immunofluorescence and confocal laser scanning microscopy was performed 10 h after seeding. Bar, 25 μm.
Figure 4
Collagen I-induced motility is dependent on a balance between cell–cell and cell– matrix interactions. C1199Tiam1-expressing MDCK-f3 cells were seeded as larger, loosely attached clusters of 5–10 cells on collagen I, to mimic conditions of high cellular density. In the absence of HGF, most cells formed E-cadherin– mediated adhesions, but some cells at the outer edge of the colony moved away from the colony (arrowhead). The presence of HGF increased motility, synergizing with the collagen effect. Immunofluorescence and confocal laser scanning microscopy was performed 10 h after seeding. Bar, 25 μm.
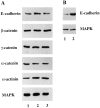
Figure 5
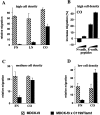
Expression of C1199Tiam1 in Ras-transformed MDCK-f3 cells increases the amount of functional E-cadherin molecules at the cell surface. (A) MDCK-f3 (lane 1) and C1199Tiam- (lane 2) or V12Rac-expressing MDCK-f3 cells (lane 3) were grown for 2 d on plastic to allow production of their own (fibronectin) matrix. Expression of E-cadherin, β-catenin, γ-catenin, α-catenin, and α-actinin was compared in cell lysates by Western blot analysis. No differences in total amount of protein could be detected. (B) MDCK-f3 (lane 1) and C1199Tiam-expressing MDCK-f3 cells (lane 2) were grown as described in (A) and subjected to cell surface biotinylation. Biotinylated E-cadherin was immunoprecipitated with avidin-agarose and detected on Western blot using an antibody against E-cadherin. Equal amounts of protein were analyzed, as determined by probing the cell lysates with an antibody against mitogen-associated protein kinase. In cells expressing Tiam1, we found a threefold increase in E-cadherin present at the cell surface.
Figure 6
Tiam1 inhibits or promotes migration of MDCK-f3 cells in Transwell assays dependent on the substrate and cell density. Migration rates of (A and B) 1.5 × 105, (C) 0.75 × 105, or (D) 0.15 × 105 cells per well were determined towards HGF contained in the lower chamber of a Transwell. (A) Migration of C1199Tiam1-expressing MDCK-f3 cells is inhibited on fibronectin (FN) and laminin 1 (LN). The migration rate on collagen I (CO) is increased as compared with the other substrates, but is still inhibited when compared with control cells. (B) Addition of increasing amounts (400 and 600 μg/ml) of HAV-peptides against E-cadherin but not N-cadherin increases migration of C1199Tiam1-expressing cells on collagen I. (C and D) Migration rates determined with lower numbers of cells. On collagen I the motile behavior of C1199Tiam1-expressing MDCK-f3 cells, as compared with control MDCK-f3 cells, increases when the cell densities in the assay are reduced (compare A, C, and D). Each bar represents the mean ± SD of triplicate migration assays. One representative example of three independent experiments is shown.
Figure 7
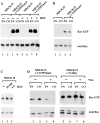
Tiam1-mediated Rac activation on fibronectin and collagen I does not differ and is dependent on PI3-kinase activity. (A) MDCK-f3 cells (lanes 1, 2, 5, and 6) or C1199Tiam1-expressing MDCK-f3 cells (lanes 3, 4, 7, and 8) were seeded in small clusters of 2–4 cells on a fibronectin (lanes 1, 3, 5, and 7) or collagen I (lanes 2, 4, 6, and 8) substrate in the absence (lanes 1–4) or presence of HGF (lanes 5–8). Cell lysates were incubated with GST–PAK-CD fusion protein and bound, active GTP–Rac molecules were analyzed by Western blotting. Cell lysates probed for total Rac are shown as a control. (B) To exclude effects of cell density on the Rac activity assay, MDCK-f3 cells (lanes 1 and 2) or C1199Tiam1-expressing MDCK-f3 cells (lanes 3 and 4) were seeded in small clusters (2–4 cells) in a four- to fivefold lower density as in (A) on a fibronectin (lanes 1 and 3) or collagen I (lanes 2 and 4) substrate. Cell lysates were analyzed as described in A. (C) Rac activities of wild-type epithelial MDCKII cells in the absence (lane 1) or presence of HGF for the indicated time (lanes 2 and 3). Cell lysates were analyzed as described in A. (D) Rac activation in C1199Tiam1- (lanes 1–4) or V12Rac-expressing MDCK-f3 cells (lanes 5–8) in the absence (lanes 1, 2, 5, and 6) or the presence (lanes 3, 4, 7, and 8) of the PI3-kinase inhibitor wortmannin (50 nM). Cells were seeded on a fibronectin (lanes 1, 3, 5, and 7) or collagen I (lanes 2, 4, 6, and 8) substrate and cell lysates were analyzed as described in A. The Myc tag of V12Rac increases the apparent molecular weight of this protein and V12Rac exhibits therefore a slightly lower electrophoretic mobility than endogenous Rac. In cell lysates, Myc-tagged V12Rac as well as endogenous Rac are detected by the antibody against Rac (lanes 5–8).
Figure 8
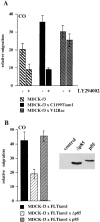
Tiam1- but not V12Rac- stimulated motility is dependent on PI3-kinase. (A) Migration rates of MDCK-f3 cells, C1199Tiam1-, or V12Rac-expressing cells were determined on collagen I towards HGF contained in the lower chamber of a Transwell (0.25 × 105 cells per well), either in the absence or presence of the PI3-kinase inhibitor LY294002 (15 μM). (B) Migration rates of FLTiam1-expressing MDCK-f3 cells (0.25 × 105 cells per well) transduced either with the control empty vector or with constructs encoding the PI3-kinase subunit wild-type p85 or dominant-negative Δp85. Both Δp85 and p85 subunits were expressed, as determined by immunoprecipitation (from 10 × 106 cells) with 12CA5 antibody against the hemagglutinin epitope of p85, followed by Western blot analysis of the precipitates with a monoclonal antibody against p85. In contrast to wild-type p85, dominant-negative Δp85 inhibited Tiam1-mediated migration twofold. Each bar represents the mean ± SD of triplicate migration assays. One representative example of three independent experiments is shown. (C) Coexpression of Δp85 but not p85 in FLTiam1-expressing MDCK-f3 cells inhibits the migratory, polarized phenotype of these cells on collagen I. Small clusters (2–4 cells) of trypsinized MDCK-f3 cells coexpressing FLTiam1 and either Δp85 or p85 were seeded in the presence of HGF onto collagen I and photographed after 4 h.
Figure 8
Tiam1- but not V12Rac- stimulated motility is dependent on PI3-kinase. (A) Migration rates of MDCK-f3 cells, C1199Tiam1-, or V12Rac-expressing cells were determined on collagen I towards HGF contained in the lower chamber of a Transwell (0.25 × 105 cells per well), either in the absence or presence of the PI3-kinase inhibitor LY294002 (15 μM). (B) Migration rates of FLTiam1-expressing MDCK-f3 cells (0.25 × 105 cells per well) transduced either with the control empty vector or with constructs encoding the PI3-kinase subunit wild-type p85 or dominant-negative Δp85. Both Δp85 and p85 subunits were expressed, as determined by immunoprecipitation (from 10 × 106 cells) with 12CA5 antibody against the hemagglutinin epitope of p85, followed by Western blot analysis of the precipitates with a monoclonal antibody against p85. In contrast to wild-type p85, dominant-negative Δp85 inhibited Tiam1-mediated migration twofold. Each bar represents the mean ± SD of triplicate migration assays. One representative example of three independent experiments is shown. (C) Coexpression of Δp85 but not p85 in FLTiam1-expressing MDCK-f3 cells inhibits the migratory, polarized phenotype of these cells on collagen I. Small clusters (2–4 cells) of trypsinized MDCK-f3 cells coexpressing FLTiam1 and either Δp85 or p85 were seeded in the presence of HGF onto collagen I and photographed after 4 h.
Figure 9
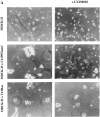
Tiam1- but not V12Rac-mediated cell–cell adhesion is dependent on PI3-kinase activity. (A) Dissociation assay with MDCK-f3 cells and C1199Tiam1- or V12Rac-expressing cells, either in the presence or absence of the PI3-kinase inhibitor LY294002 (25 μM). In contrast to control MDCK-f3 cells, cells expressing C1199Tiam1 form large aggregates. Note that aggregates induced by expression of V12Rac are smaller compared with those observed with C1199Tiam1, consistent with the findings that the phenotypic reversion induced with V12Rac is less pronounced as the Tiam1-induced reversion (Fig. 1). Upon inhibition of PI3-kinase, cell aggregates induced by expression of C1199Tiam1 decrease in size, whereas aggregates induced by expression of V12Rac are not dependent on PI3-kinase activity. (B) Quantification of cell–cell adhesion of the MDCK-f3 cell lines determined by dissociation assays and expressed as numbers of particles (cell clusters) per total number of cells (Np/Nc). Each bar represents the mean ± SD of triplicate dissociation assays. One representative example of four independent experiments is shown.
Figure 9
Tiam1- but not V12Rac-mediated cell–cell adhesion is dependent on PI3-kinase activity. (A) Dissociation assay with MDCK-f3 cells and C1199Tiam1- or V12Rac-expressing cells, either in the presence or absence of the PI3-kinase inhibitor LY294002 (25 μM). In contrast to control MDCK-f3 cells, cells expressing C1199Tiam1 form large aggregates. Note that aggregates induced by expression of V12Rac are smaller compared with those observed with C1199Tiam1, consistent with the findings that the phenotypic reversion induced with V12Rac is less pronounced as the Tiam1-induced reversion (Fig. 1). Upon inhibition of PI3-kinase, cell aggregates induced by expression of C1199Tiam1 decrease in size, whereas aggregates induced by expression of V12Rac are not dependent on PI3-kinase activity. (B) Quantification of cell–cell adhesion of the MDCK-f3 cell lines determined by dissociation assays and expressed as numbers of particles (cell clusters) per total number of cells (Np/Nc). Each bar represents the mean ± SD of triplicate dissociation assays. One representative example of four independent experiments is shown.
Similar articles
- Inhibition of invasion of epithelial cells by Tiam1-Rac signaling.
Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Hordijk PL, et al. Science. 1997 Nov 21;278(5342):1464-6. doi: 10.1126/science.278.5342.1464. Science. 1997. PMID: 9367959 - The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions.
Malliri A, van Es S, Huveneers S, Collard JG. Malliri A, et al. J Biol Chem. 2004 Jul 16;279(29):30092-8. doi: 10.1074/jbc.M401192200. Epub 2004 May 11. J Biol Chem. 2004. PMID: 15138270 - Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior.
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Sander EE, et al. J Cell Biol. 1999 Nov 29;147(5):1009-22. doi: 10.1083/jcb.147.5.1009. J Cell Biol. 1999. PMID: 10579721 Free PMC article. - The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression.
Minard ME, Kim LS, Price JE, Gallick GE. Minard ME, et al. Breast Cancer Res Treat. 2004 Mar;84(1):21-32. doi: 10.1023/B:BREA.0000018421.31632.e6. Breast Cancer Res Treat. 2004. PMID: 14999151 Review. - Phosphatidylinositol 3-kinase: a key regulator in adherens junction formation and function.
Rivard N. Rivard N. Front Biosci (Landmark Ed). 2009 Jan 1;14(2):510-22. doi: 10.2741/3259. Front Biosci (Landmark Ed). 2009. PMID: 19273082 Review.
Cited by
- SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline.
Guéguinou M, Harnois T, Crottes D, Uguen A, Deliot N, Gambade A, Chantôme A, Haelters JP, Jaffrès PA, Jourdan ML, Weber G, Soriani O, Bougnoux P, Mignen O, Bourmeyster N, Constantin B, Lecomte T, Vandier C, Potier-Cartereau M. Guéguinou M, et al. Oncotarget. 2016 Jun 14;7(24):36168-36184. doi: 10.18632/oncotarget.8786. Oncotarget. 2016. PMID: 27102434 Free PMC article. - Shc and FAK differentially regulate cell motility and directionality modulated by PTEN.
Gu J, Tamura M, Pankov R, Danen EH, Takino T, Matsumoto K, Yamada KM. Gu J, et al. J Cell Biol. 1999 Jul 26;146(2):389-403. doi: 10.1083/jcb.146.2.389. J Cell Biol. 1999. PMID: 10427092 Free PMC article. - The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex.
Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. Mertens AE, et al. J Cell Biol. 2005 Sep 26;170(7):1029-37. doi: 10.1083/jcb.200502129. J Cell Biol. 2005. PMID: 16186252 Free PMC article. - The Borgs, a new family of Cdc42 and TC10 GTPase-interacting proteins.
Joberty G, Perlungher RR, Macara IG. Joberty G, et al. Mol Cell Biol. 1999 Oct;19(10):6585-97. doi: 10.1128/MCB.19.10.6585. Mol Cell Biol. 1999. PMID: 10490598 Free PMC article. - Role of protein tyrosine phosphatase SHP2 in barrier function of pulmonary endothelium.
Grinnell KL, Casserly B, Harrington EO. Grinnell KL, et al. Am J Physiol Lung Cell Mol Physiol. 2010 Mar;298(3):L361-70. doi: 10.1152/ajplung.00374.2009. Epub 2009 Dec 18. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20023173 Free PMC article.
References
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. - PubMed
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous