Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity - PubMed (original) (raw)
Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity
P A Stumbles et al. J Exp Med. 1998.
Abstract
Consistent with their role in host defense, mature dendritic cells (DCs) from central lymphoid organs preferentially prime for T helper cell type 1 (Th1)-polarized immunity. However, the "default" T helper response at mucosal surfaces demonstrates Th2 polarity, which is reflected in the cytokine profiles of activated T cells from mucosal lymph nodes. This study on rat respiratory tract DCs (RTDCs) provides an explanation for this paradox. We demonstrate that freshly isolated RTDCs are functionally immature as defined in vitro, being surface major histocompatibility complex (MHC) II lo, endocytosishi, and mixed lymphocyte reactionlo, and these cells produce mRNA encoding interleukin (IL)-10. After ovalbumin (OVA)-pulsing and adoptive transfer, freshly isolated RTDCs preferentially stimulated Th2-dependent OVA-specific immunoglobulin (Ig)G1 responses, and antigen-stimulated splenocytes from recipient animals produced IL-4 in vitro. However, preculture with granulocyte/macrophage colony stimulating factor increased their in vivo IgG priming capacity by 2-3 logs, inducing production of both Th1- and Th2-dependent IgG subclasses and high levels of IFN-gamma by antigen-stimulated splenocytes. Associated phenotypic changes included upregulation of surface MHC II and B7 expression and IL-12 p35 mRNA, and downregulation of endocytosis, MHC II processing- associated genes, and IL-10 mRNA expression. Full expression of IL-12 p40 required additional signals, such as tumor necrosis factor alpha or CD40 ligand. These results suggest that the observed Th2 polarity of the resting mucosal immune system may be an inherent property of the resident DC population, and furthermore that mobilization of Th1 immunity relies absolutely on the provision of appropriate microenvironmental costimuli.
Figures
Figure 1
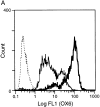
Surface MHC class II expression and MLR-stimulating activity of fresh and GM-CSF–exposed RTDCs. (A) Purified RTDCs were labeled with OX6-FITC either as fresh cells (thin line) or after culture in GM-CSF (thick line), or with the isotype control IgG1-FITC (dotted line), and surface fluorescence analyzed by flow cytometry. (B) Serial dilutions of freshly purified (open circles) or GM-CSF–exposed (filled circles) RTDCs were added as stimulators to a primary allogeneic MLR in the presence of 105 purified WAG-strain T cells per well of 96-well microtiter plates. Cell proliferation (CPM) was assessed after a total of 96 h including an 18-h [3H]TdR pulse. Mean ± SEM of triplicate wells of one representative of three experiments is shown.
Figure 1
Surface MHC class II expression and MLR-stimulating activity of fresh and GM-CSF–exposed RTDCs. (A) Purified RTDCs were labeled with OX6-FITC either as fresh cells (thin line) or after culture in GM-CSF (thick line), or with the isotype control IgG1-FITC (dotted line), and surface fluorescence analyzed by flow cytometry. (B) Serial dilutions of freshly purified (open circles) or GM-CSF–exposed (filled circles) RTDCs were added as stimulators to a primary allogeneic MLR in the presence of 105 purified WAG-strain T cells per well of 96-well microtiter plates. Cell proliferation (CPM) was assessed after a total of 96 h including an 18-h [3H]TdR pulse. Mean ± SEM of triplicate wells of one representative of three experiments is shown.
Figure 2
CD80 and CD86 expression by fresh and GM-CSF–exposed RTDCs. Fresh (A) or GM-CSF–exposed (B) RTDC were colabeled with the OX21 (IgG1), 3H5 (CD80), or 24F (CD86) mAbs plus GAM-PE followed by OX6-FITC, and surface fluorescence analyzed by flow cytometry. Quadrant markers were set for IgG1-FITC/IgG1 plus GAM-PE negative control cells.
Figure 3
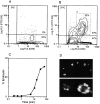
Endocytic activity of fresh RTDC. Freshly purified RTDC were incubated at 0°C (A) or 37°C (B) for 90 min in the presence of 0.5 mg/ml FITC-DX then labeled with the OX6 mAb plus GAM-PE and fluorescence levels analyzed by flow cytometry after trypan blue quenching. (C) Time-course of FITC-DX uptake by fresh RTDCs. RTDCs were incubated with FITC-DX as described above and the percentage of endocytically positive cells falling within an arbitrary gating region (B, a) determined by flow cytometry. Percentage endocytic values were calculated by subtracting values obtained at 0°C from those obtained at 37°C. One representative out of three experiments is shown. (D) Confocal microscopic analysis of FITC-DX uptake by fresh RTDCs.
Figure 4
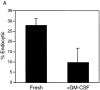
Inhibition of the endocytic activity of fresh RTDCs by GM-CSF. Fresh or GM-CSF–exposed RTDCs were incubated for 90 min at 37°C with 0.5 mg/ml FITC-DX. Percentage endocytic (A) or Δ MFI (B) values were then calculated from 0°C and 37°C percentage and mean fluorescence values using gating as described in Fig. 3_B_. Mean values ± SEM for three experiments are shown.
Figure 4
Inhibition of the endocytic activity of fresh RTDCs by GM-CSF. Fresh or GM-CSF–exposed RTDCs were incubated for 90 min at 37°C with 0.5 mg/ml FITC-DX. Percentage endocytic (A) or Δ MFI (B) values were then calculated from 0°C and 37°C percentage and mean fluorescence values using gating as described in Fig. 3_B_. Mean values ± SEM for three experiments are shown.
Figure 5
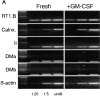
Changes in expression of mRNA encoding components of the MHC class II biosynthesis/processing pathway in fresh or GM-CSF– exposed RTDC. (A) cDNA was prepared from RTDCs either as fresh cells or after overnight culture in GM-CSF and used either undiluted (undil.) or diluted 1:5 or 1:25 in a PCR reaction using primers specific for the indicated MHC class II biosynthesis and antigen processing-associated proteins as described in Materials and Methods. (B) MHC class II component:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh (solid bars) and GM-CSF–exposed (white bars) RTDCs. One representative out of two experiments is shown. RT1.B, rat MHC class II; Calnx, calnexin; Ii, invariant chain; DMa/DMb, RT1.DMa/b.
Figure 5
Changes in expression of mRNA encoding components of the MHC class II biosynthesis/processing pathway in fresh or GM-CSF– exposed RTDC. (A) cDNA was prepared from RTDCs either as fresh cells or after overnight culture in GM-CSF and used either undiluted (undil.) or diluted 1:5 or 1:25 in a PCR reaction using primers specific for the indicated MHC class II biosynthesis and antigen processing-associated proteins as described in Materials and Methods. (B) MHC class II component:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh (solid bars) and GM-CSF–exposed (white bars) RTDCs. One representative out of two experiments is shown. RT1.B, rat MHC class II; Calnx, calnexin; Ii, invariant chain; DMa/DMb, RT1.DMa/b.
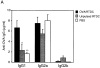
Figure 6
In vivo priming for OVA-specific IgG production by freshly isolated RTDCs. Freshly sorted RTDCs were pulsed with 1 mg/ml OVA for 90 min at 37°C and washed, and 105 cells in 1 ml PBS were transferred intravenously into syngeneic recipients. (A) Animals received a single dose of OVA-pulsed RTDCs on day 0 and were then challenged with 10 μg/ml OVA on day 5 and bled on day 20. Control animals received either 105 unpulsed RTDCs or 1 ml PBS on day 0. *P = 0.01 and **P = 0.005 compared with OVA-pulsed RTDCs. (B) Animals received one dose of RTDCs weekly for 3 wk and were bled 1 wk after final RTDC transfer. *P = 0.005; **P = 0.008; and ***P = 0.01 compared with animals receiving 1× RTDCs. In all cases serum OVA-specific IgG1, 2a, and 2b levels were then assessed by ELISA as described in Materials and Methods. Mean ± SEM of five animals in each group are shown and these experiments have been repeated on several subsequent occasions.
Figure 6
In vivo priming for OVA-specific IgG production by freshly isolated RTDCs. Freshly sorted RTDCs were pulsed with 1 mg/ml OVA for 90 min at 37°C and washed, and 105 cells in 1 ml PBS were transferred intravenously into syngeneic recipients. (A) Animals received a single dose of OVA-pulsed RTDCs on day 0 and were then challenged with 10 μg/ml OVA on day 5 and bled on day 20. Control animals received either 105 unpulsed RTDCs or 1 ml PBS on day 0. *P = 0.01 and **P = 0.005 compared with OVA-pulsed RTDCs. (B) Animals received one dose of RTDCs weekly for 3 wk and were bled 1 wk after final RTDC transfer. *P = 0.005; **P = 0.008; and ***P = 0.01 compared with animals receiving 1× RTDCs. In all cases serum OVA-specific IgG1, 2a, and 2b levels were then assessed by ELISA as described in Materials and Methods. Mean ± SEM of five animals in each group are shown and these experiments have been repeated on several subsequent occasions.
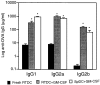
Figure 7
In vivo priming for OVA-specific IgG production by GM-CSF–exposed RTDCs. Sorted RTDCs or splenic DCs (SpDC) were incubated overnight at 37°C with 1 mg/ml OVA and 10 ng/ml GM-CSF, then transferred intravenously to syngeneic recipients, and serum IgG1, 2a, and 2b levels were determined as described in Fig. 6_A_. Identical results for RTDCs were obtained using 90-min OVA-pulsing before overnight culture in GM-CSF (data not shown). Results are expressed as mean ± SEM for 9 (RTDC) or 10 (SpDC) animals. *P < 0.0001 compared with fresh RTDCs.
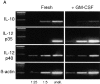
Figure 8
Expression of IL-10 and IL-12 by fresh and GM-CSF– exposed RTDCs. (A) cDNA prepared from freshly isolated or GM-CSF– exposed RTDCs was used either undiluted (undil.) or diluted 1:5 and 1:25 in PCR reactions for rat IL-10 and IL-12p35 and p40 mRNA as described in Materials and Methods. (B) IL-10/IL-12:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh RTDCs and RTDCs exposed to GM-CSF alone for 24 or 48 h or GM-CSF plus TNF or CD40L for 48 h only. Mean values ± SEM for three experiments are shown for fresh and GM-CSF–exposed RTDCs, and representative results of a single experiment are shown for GM-CSF plus TNF or CD40L.
Figure 8
Expression of IL-10 and IL-12 by fresh and GM-CSF– exposed RTDCs. (A) cDNA prepared from freshly isolated or GM-CSF– exposed RTDCs was used either undiluted (undil.) or diluted 1:5 and 1:25 in PCR reactions for rat IL-10 and IL-12p35 and p40 mRNA as described in Materials and Methods. (B) IL-10/IL-12:β-actin mRNA band density ratios (1:5 cDNA dilution) for fresh RTDCs and RTDCs exposed to GM-CSF alone for 24 or 48 h or GM-CSF plus TNF or CD40L for 48 h only. Mean values ± SEM for three experiments are shown for fresh and GM-CSF–exposed RTDCs, and representative results of a single experiment are shown for GM-CSF plus TNF or CD40L.
Similar articles
- Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses.
Kelsall BL, Stüber E, Neurath M, Strober W. Kelsall BL, et al. Ann N Y Acad Sci. 1996 Oct 31;795:116-26. doi: 10.1111/j.1749-6632.1996.tb52660.x. Ann N Y Acad Sci. 1996. PMID: 8958922 - The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo.
Ritz SA, Cundall MJ, Gajewska BU, Swirski FK, Wiley RE, Alvarez D, Coyle AJ, Stampfli MR, Jordana M. Ritz SA, et al. Clin Exp Immunol. 2004 Nov;138(2):213-20. doi: 10.1111/j.1365-2249.2004.02618.x. Clin Exp Immunol. 2004. PMID: 15498029 Free PMC article. - Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells.
Ria F, Penna G, Adorini L. Ria F, et al. Eur J Immunol. 1998 Jun;28(6):2003-16. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. Eur J Immunol. 1998. PMID: 9645382 - Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.
Schülke S. Schülke S. Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review. - Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells.
Onoé K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Onoé K, et al. Immunol Res. 2007;38(1-3):319-32. doi: 10.1007/s12026-007-0011-5. Immunol Res. 2007. PMID: 17917039 Review.
Cited by
- Oral drug delivery for immunoengineering.
Le T, Aguilar B, Mangal JL, Acharya AP. Le T, et al. Bioeng Transl Med. 2021 Aug 10;7(1):e10243. doi: 10.1002/btm2.10243. eCollection 2022 Jan. Bioeng Transl Med. 2021. PMID: 35111945 Free PMC article. Review. - Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection.
Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Khader SA, et al. J Exp Med. 2006 Jul 10;203(7):1805-15. doi: 10.1084/jem.20052545. Epub 2006 Jul 3. J Exp Med. 2006. PMID: 16818672 Free PMC article. - Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice.
Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, Van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Bedoret D, et al. J Clin Invest. 2009 Dec;119(12):3723-38. doi: 10.1172/JCI39717. Epub 2009 Nov 9. J Clin Invest. 2009. PMID: 19907079 Free PMC article. - Molecular mechanisms of atopy.
Barnes PJ. Barnes PJ. Mediators Inflamm. 2001 Dec;10(6):285-8. doi: 10.1080/09629350120102000. Mediators Inflamm. 2001. PMID: 11817661 Free PMC article. Review. No abstract available. - Production of interleukin (IL)-10 and IL-12 by murine colonic dendritic cells in response to microbial stimuli.
Rigby RJ, Knight SC, Kamm MA, Stagg AJ. Rigby RJ, et al. Clin Exp Immunol. 2005 Feb;139(2):245-56. doi: 10.1111/j.1365-2249.2004.02674.x. Clin Exp Immunol. 2005. PMID: 15654823 Free PMC article.
References
- Holt PG, Schon-Hegrad MA, Phillips MJ, McMenamin PG. Ia-positive dendritic cells form a tightly meshed network within the human airway epithelium. Clin Exp Allergy. 1989;19:597–601. - PubMed
- Nicod LP, Lipscomb MF, Weissler JC, Lyons CR, Albertson J, Toews GB. Mononuclear cells in human lung parenchyma. Characterization of a potent accessory cell not obtained by bronchoalveolar lavage. Am Rev Respir Dis. 1987;136:818–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials