Production of beta-defensins by human airway epithelia - PubMed (original) (raw)
. 1998 Dec 8;95(25):14961-6.
doi: 10.1073/pnas.95.25.14961.
H P Jia, K Wiles, J Hesselberth, L Liu, B A Conway, E P Greenberg, E V Valore, M J Welsh, T Ganz, B F Tack, P B McCray Jr
Affiliations
- PMID: 9843998
- PMCID: PMC24558
- DOI: 10.1073/pnas.95.25.14961
Production of beta-defensins by human airway epithelia
P K Singh et al. Proc Natl Acad Sci U S A. 1998.
Erratum in
- Proc Natl Acad Sci U S A 1999 Mar 2;96(5):2569
Abstract
Human beta-defensins (HBDs) are antimicrobial peptides that may play a role in mucosal defense. Diminished activity of these peptides has been implicated in the pathogenesis of cystic fibrosis (CF) lung disease. We show that HBD-1 and HBD-2 mRNAs are expressed in excised surface and submucosal gland epithelia from non-CF and CF patients. The pro-inflammatory cytokine interleukin-1beta stimulated the expression of HBD-2 but not HBD-1 mRNA and peptide in primary cultures of airway epithelia. HBD-1 was found in bronchoalveolar lavage (BAL) fluid from normal volunteers, CF patients, and patients with inflammatory lung diseases, whereas HBD-2 was detected in BAL fluid from patients with CF or inflammatory lung diseases, but not in normal volunteers. Both HBD-1 and HBD-2 were found in BAL fluid in concentrations of several ng/ml, and both recombinant peptides showed salt-sensitive bactericidal activity. These data suggest that in the lung HBD-2 expression is induced by inflammation, whereas HBD-1 may serve as a defense in the absence of inflammation.
Figures
Figure 1
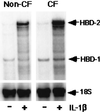
Expression of HBD-1 and HBD-2 mRNAs in cultured normal and CF airway epithelia measured by using the ribonuclease protection assay. Cells were studied under basal conditions (−) and after 24 h of IL-1β treatment (+; 100 ng/ml). For both non-CF and CF specimens, constitutive HBD-1 mRNA expression was detected and was unchanged by IL-1β treatment. In contrast, the mRNA for HBD-2 was markedly induced after IL-1β treatment. 18S rRNA was used as an internal control to standardize the mRNA signal. Shown is a representative assay from three separate experiments with similar results.
Figure 2
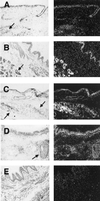
Localization of β-defensin mRNA expression in normal and CF airway by in situ hybridization. For each pair of images the left-hand panel is a bright-field image and the right-hand panel is dark-field. (A) HBD-1 is expressed in normal human bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (B) HBD-1 is also expressed in CF bronchus in surface and submucosal gland epithelia (antisense probe, 11-week exposure). (C) HBD-2 is expressed in normal human bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (D) HBD-2 is expressed in CF bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (E) Representative control sense riboprobe for HBD-1 with nonspecific background. Similar results were found with the HBD-2 sense riboprobe. Arrows denote submucosal glands. (×10.)
Figure 3
Detection of HBD-1 and HBD-2 proteins in non-CF and CF ASL by acid/urea/PAGE and immunoblotting. Epithelia were cultured with (+) and without (−) stimulation. (Upper) Comparison of HBD-1 peptide abundance in non-CF and CF epithelia. No HBD-1 peptide was found in washings from non-CF or CF epithelia, but 5 ng of recombinant HBD-1 peptide (STD) was easily detected. No HBD-1 was detected in the cell lysates (not shown). (Lower) Comparison of HBD-2 abundance in non-CF and CF epithelia. The apical surface was washed with water to remove accumulated peptide prior to IL-1β treatment. HBD-2 peptide was easily detected in the washings from non-CF and CF epithelia. Little peptide was recovered with the subsequent NH4OAc wash, indicating good recovery of protein with aqueous washes (not shown). HBD-2 was also detected in the cell lysate (not shown). With IL-1β stimulation, protein recovery increased in all fractions. Results for CF cells are qualitatively similar to those from cultured non-CF airway epithelia. Control was 7.5 ng of HBD-2. Representative results are shown.
Figure 4
Concentration of β-defensin peptides in BAL fluid measured by Western immunoblotting. Human BAL samples were obtained and analyzed by acid/urea/PAGE and Western immunoblotting with specific antisera for HBD-1 and HBD-2. Samples from non-CF (NL), CF, and patients with inflammatory lung diseases (ILD) were studied. (A) Representative immunoblot results for lysozyme (LYSO), HBD-1, and HBD-2 (BAL, patient sample; Std, protein standard). (B) BAL β-defensin concentrations determined by comparison to peptide standards. Each open circle represents the value from a single patient sample. HBD-1 was detectable in some samples from all three groups but there were no statistically significant differences among the groups. HBD-2 was detected only in BAL from patients with CF or ILD (P < 0.05 for both groups compared with non-CF by ANOVA).
Figure 5
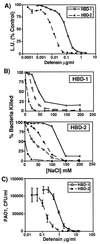
Antimicrobial activity and salt sensitivity of recombinant HBD-1 and HBD-2 peptides. (A) E. coli luminescence assay. HBD-1 and HBD-2 both kill E. coli in a dose-dependent fashion. The activity of HBD-2 was ≈10-fold greater than that of HBD-1. Each point represents mean ± SE for triplicate samples. (B) Salt sensitivity of HBD-1 and HBD-2. Increasing concentrations of NaCl inhibit the activity of both peptides against E. coli. The degree of inhibition by salt is also dependent on the concentration of peptide. For HBD-1, ○ = 12 μg/ml, □ = 6 μg/ml, and ⋄ = 1.2 μg/ml. For HBD-2, ○ = 0.5 μg/ml, ⋄ = 0.1 μg/ml, □ = 0.05 μg/ml, and ▵, 0.01 μg/ml. Results were replicated twice. (C) Colony-forming unit (CFU) assay for killing of P. aeruginosa by HBD-1 and HBD-2. Each point represents mean ± SE for triplicate samples.
Similar articles
- Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis.
Hiratsuka T, Mukae H, Iiboshi H, Ashitani J, Nabeshima K, Minematsu T, Chino N, Ihi T, Kohno S, Nakazato M. Hiratsuka T, et al. Thorax. 2003 May;58(5):425-30. doi: 10.1136/thorax.58.5.425. Thorax. 2003. PMID: 12728165 Free PMC article. - Human airway epithelia express a beta-defensin.
McCray PB Jr, Bentley L. McCray PB Jr, et al. Am J Respir Cell Mol Biol. 1997 Mar;16(3):343-9. doi: 10.1165/ajrcmb.16.3.9070620. Am J Respir Cell Mol Biol. 1997. PMID: 9070620 - Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung.
Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Bals R, et al. J Clin Invest. 1998 Sep 1;102(5):874-80. doi: 10.1172/JCI2410. J Clin Invest. 1998. PMID: 9727055 Free PMC article. - Epithelial antimicrobial peptides: review and significance for oral applications.
Weinberg A, Krisanaprakornkit S, Dale BA. Weinberg A, et al. Crit Rev Oral Biol Med. 1998;9(4):399-414. doi: 10.1177/10454411980090040201. Crit Rev Oral Biol Med. 1998. PMID: 9825219 Review. - [Enhancement of BCG-induced hBD-1 mRNA expression in human pulmonary gland epithelial cells].
Feng Y, Huang N, Wu Q, Wang B. Feng Y, et al. Hua Xi Yi Ke Da Xue Xue Bao. 2000 Sep;31(3):285-8. Hua Xi Yi Ke Da Xue Xue Bao. 2000. PMID: 12545809 Review. Chinese.
Cited by
- IL-13 induces loss of CFTR in ionocytes and reduces airway epithelial fluid absorption.
Romano Ibarra GS, Lei L, Yu W, Thurman AL, Gansemer ND, Meyerholz DK, Pezzulo AA, McCray PB, Thornell IM, Stoltz DA. Romano Ibarra GS, et al. J Clin Invest. 2024 Sep 10;134(21):e181995. doi: 10.1172/JCI181995. J Clin Invest. 2024. PMID: 39255033 Free PMC article. - CFTR dysfunction leads to defective bacterial eradication on cystic fibrosis airways.
Wu M, Chen JH. Wu M, et al. Front Physiol. 2024 Apr 18;15:1385661. doi: 10.3389/fphys.2024.1385661. eCollection 2024. Front Physiol. 2024. PMID: 38699141 Free PMC article. Review. - Airway Epithelial-Derived Immune Mediators in COVID-19.
Guo TJF, Singhera GK, Leung JM, Dorscheid DR. Guo TJF, et al. Viruses. 2023 Jul 29;15(8):1655. doi: 10.3390/v15081655. Viruses. 2023. PMID: 37631998 Free PMC article. Review. - Serum β-Defensin 2, A Novel Biomarker for the Diagnosis of Acute Infections.
Routsias JG, Marinou D, Mavrouli M, Tsakris A, Pitiriga V. Routsias JG, et al. Diagnostics (Basel). 2023 May 28;13(11):1885. doi: 10.3390/diagnostics13111885. Diagnostics (Basel). 2023. PMID: 37296737 Free PMC article. - Recombinant human β-defensin130 inhibited the growth of foodborne bacteria through membrane disruption and exerted anti-inflammatory activity.
Dong B, Lin Y, Su Z, Sun C, Wang J, Fu S, Du W, Wu T. Dong B, et al. Food Sci Biotechnol. 2022 Apr 20;31(7):893-904. doi: 10.1007/s10068-022-01087-y. eCollection 2022 Jul. Food Sci Biotechnol. 2022. PMID: 35720462 Free PMC article.
References
- Goldman M J, Anderson M G, Stolzenberg E D, Kari P U, Zasloff M, Wilson J M. Cell. 1997;88:1–9. - PubMed
- Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. FEBS Lett. 1995;368:331–335. - PubMed
- Harder J, Bartels J, Christophers E, Schroder J-M. Nature (London) 1997;387:861–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources