Nucleoporins nup98 and nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev - PubMed (original) (raw)
Nucleoporins nup98 and nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev
A S Zolotukhin et al. J Virol. 1999 Jan.
Abstract
Human immunodeficiency virus type 1 (HIV-1) Rev contains a leucine-rich nuclear export signal that is essential for its nucleocytoplasmic export mediated by hCRM1. We examined the role of selected nucleoporins, which are located in peripheral structures of the nuclear pore complex and are thought to be involved in export, in Rev function in human cells. First, we found that upon actinomycin D treatment, Nup98, but not Nup214 or Nup153, is able to translocate to the cytoplasm of HeLa cells, demonstrating that Nup98 may act as a soluble factor. We further showed that Rev can recruit Nup98 and Nup214, but not Nup153, to the nucleolus. We also found that the isolated FG-containing repeat domains of Nup98 and Nup214, but not those of Nup153, competitively inhibit the Rev/RRE-mediated expression of HIV. Taken together, the recruitment of Nup98 and Nup214 by Rev and the competitive inhibition exhibited by their NP domains demonstrate direct participation of Nup98 and Nup214 in the Rev-hCRM1-mediated export.
Figures
FIG. 1
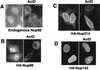
Nup98, but not Nup153 and Nup214, is able to translocate to the cytoplasm. Endogenous Nup98 was visualized by using affinity-purified anti-Xenopus Nup98 serum (A), whereas HA-tagged Nup98, Nup214, and Nup153 proteins were detected with anti-HA antibody (B to D). HLtat cells were transiently transfected with 2 μg of the indicated HA-tagged nucleoporin expression plasmids (B to D) or not transfected (A). Some cells were treated with 2 μg of actinomycin D (ActD) per ml for 2 h. The indirect immunofluorescence and fluorescence microscopy were performed as described in Materials and Methods, and the images were filtered with a linear filter (3 × 3 kernel), using IPLab Spectrum software. Bar, 20 μm.
FIG. 2
Rev recruits Nup98 and Nup214 to the nucleoli. HLtat cells were transfected with 0.5 μg of the HA-tagged nucleoporin expression plasmids in the presence of 1 μg of GFP-Rev or 1 μg of GFP-NES(−)Rev expression plasmid as indicated at the bottom of the figure. The GFP fluorescence and HA indirect immunofluorescence were detected in the same cells, as indicated to the left. Fluorescence microscopy was performed as described in the legend to Fig. 1. Bars, 20 μm.
FIG. 3
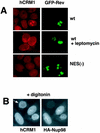
hCRM1 codistributes with Rev and Nup98. (A) Human 293 cells were transfected with 1 μg of BFP-Rev (wild type [wt]) or BFP-NES(−)Rev [NES(−)] expression plasmid as indicated to the right, and treated with 6 nM LMB for 4 h. The endogenous hCRM1 was visualized by indirect immunofluorescence with rabbit anti-hCRM1 serum (15) and rhodamine red-conjugated goat anti-rabbit secondary antibodies as previously described (43). BFP fluorescence is shown pseudocolored in green. (B) HLtat cells were transfected with 0.5 μg of HA-Nup98 and subjected to double indirect immunofluorescence for HA-Nup98 and the endogenous hCRM1, as indicated. Before immunodetection, cells were extracted in situ with 0.004% digitonin in 0.3× PBS for 10 min at 4°C. Fluorescence microscopy was performed as described in the legend to Fig. 1.
FIG. 4
NP domains of Nup98 and Nup214 specifically affect the function of Rev. (A to C) Human 293 cells were transfected with 1 μg of Rev(−)HIV in the presence of 25 ng of the pBsRev expression plasmid (Rev/RRE) or 1 μg of the Rev(−)RRE(−).CTE Rev-independent HIV-1 (CTE). All transfections included 0.1 μg of L3luc, a luciferase expression vector. Increasing amounts of expression plasmid Nup98-NP (A), Nup214-NP (B), or Nup153-NP (C) were cotransfected (plotted on the x axis in micrograms). Gag production was measured in the lysates at one day posttransfection (p24_gag_ measured in nanograms per milliliter) and is plotted on the y axis. Results from a representative experiment are shown. Similar results were obtained in three independent transfection experiments. (D and E) 293 cells were cotransfected with 1 μg of Rev(−)HIV in the presence of 25 ng of pBsRev expression plasmid and in the absence or presence of the indicated GFP-tagged NP expression plasmids. HIV expression is determined by measuring Gag production and expressed as a percentage of the value obtained in the absence of the NP domains (D). Western immunoblot analysis (E) of the extracts shown in panel D. GFP-tagged proteins were detected by using rabbit anti-GFP serum for the experiment shown in panel D. The positions of protein markers (in kilodaltons) are shown to the left. The expected molecular masses of GFP fusion proteins were 27 kDa (GFP), 77 kDa (GFP-Nup98-NP), and 86 kDa (GFP-Nup153-NP). n/t, nontransfected cells.
FIG. 5
Rev counteracts the inhibitory effect of the NP of Nup98. (A) 293 cells were transfected with 1 μg of Rev(−)HIV and 0.25 μg of pBsRev in the absence or presence of 0.25 μg of Nup98-NP. In parallel, transfections were done with 1 μg of Rev(−)RRE(−).CTE in the absence of Nup98-NP (CTE). Increasing amounts of hCRM1 expression plasmid were cotransfected (plotted on the x axis in micrograms). p24_gag_ expression was measured and is plotted on the y axis as raw values (in nanograms per milliliter of lysate). Similar results were obtained in three independent transfection experiments. Panels A to C show results from representative experiments. (B) Rev counteracts the effect of Nup98-NP on HIV-1 expression. Human 293 cells were transfected with 1 μg of Rev(−)HIV (Rev/RRE) in the presence of 0.2 μg of Nup98-NP. Transfections included the luciferase plasmid. Increasing amounts of pBsRev plasmid, starting at 25 ng, were cotransfected (plotted on the x axis in micrograms). p24_gag_ expression (in nanograms per milliliter of lysate) is plotted on the left y axis as raw values. Luciferase values are plotted on the right y axis as multiples of 103 firefly units (FFU). (C) 293 cells were cotransfected with 0.25 or 2 μg of GFP-Rev expression plasmid in the presence or absence of 0.2 μg of Nup98-NP plasmid, as indicated. At day 1 posttransfection, some cells were treated with actinomycin D (+) in the presence of cycloheximide as indicated at the top, and GFP fluorescence was visualized as described previously (43).
Similar articles
- Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin.
Hofmann W, Reichart B, Ewald A, Müller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle MC. Hofmann W, et al. J Cell Biol. 2001 Mar 5;152(5):895-910. doi: 10.1083/jcb.152.5.895. J Cell Biol. 2001. PMID: 11238447 Free PMC article. - Epstein-Barr virus EB2 protein exports unspliced RNA via a Crm-1-independent pathway.
Farjot G, Buisson M, Duc Dodon M, Gazzolo L, Sergeant A, Mikaelian I. Farjot G, et al. J Virol. 2000 Jul;74(13):6068-76. doi: 10.1128/jvi.74.13.6068-6076.2000. J Virol. 2000. PMID: 10846090 Free PMC article. - The HIV-1 Rev protein.
Pollard VW, Malim MH. Pollard VW, et al. Annu Rev Microbiol. 1998;52:491-532. doi: 10.1146/annurev.micro.52.1.491. Annu Rev Microbiol. 1998. PMID: 9891806 Review. - Retroviruses as model systems for the study of nuclear RNA export pathways.
Cullen BR. Cullen BR. Virology. 1998 Sep 30;249(2):203-10. doi: 10.1006/viro.1998.9331. Virology. 1998. PMID: 9791012 Review. No abstract available.
Cited by
- Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin.
Hofmann W, Reichart B, Ewald A, Müller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle MC. Hofmann W, et al. J Cell Biol. 2001 Mar 5;152(5):895-910. doi: 10.1083/jcb.152.5.895. J Cell Biol. 2001. PMID: 11238447 Free PMC article. - Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals.
Iarovaia OV, Ioudinkova ES, Velichko AK, Razin SV. Iarovaia OV, et al. Cells. 2021 Jun 25;10(7):1597. doi: 10.3390/cells10071597. Cells. 2021. PMID: 34202380 Free PMC article. Review. - Analysis of cellular factors that mediate nuclear export of RNAs bearing the Mason-Pfizer monkey virus constitutive transport element.
Kang Y, Bogerd HP, Cullen BR. Kang Y, et al. J Virol. 2000 Jul;74(13):5863-71. doi: 10.1128/jvi.74.13.5863-5871.2000. J Virol. 2000. PMID: 10846066 Free PMC article. - Host cell factors in HIV replication: meta-analysis of genome-wide studies.
Bushman FD, Malani N, Fernandes J, D'Orso I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, König R, Bandyopadhyay S, Ideker T, Goff SP, Krogan NJ, Frankel AD, Young JA, Chanda SK. Bushman FD, et al. PLoS Pathog. 2009 May;5(5):e1000437. doi: 10.1371/journal.ppat.1000437. Epub 2009 May 29. PLoS Pathog. 2009. PMID: 19478882 Free PMC article. Review. - Proteomics in the investigation of HIV-1 interactions with host proteins.
Li M. Li M. Proteomics Clin Appl. 2015 Feb;9(1-2):221-34. doi: 10.1002/prca.201400101. Epub 2015 Jan 19. Proteomics Clin Appl. 2015. PMID: 25523935 Free PMC article. Review.
References
- Arai Y, Hosoda F, Kobayashi H, Arai K, Hayashi Y, Kamada N, Kaneko Y, Ohki M. The inv(11)(p15q22) chromosome translocation of de novo and therapy-related myeloid malignancies results in fusion of the nucleoporin gene, NUP98, with the putative RNA helicase gene, DDX10. Blood. 1997;89:3936–3944. - PubMed
- Boer J M, van Deursen J M, Croes H J, Fransen J A, Grosveld G C. The nucleoporin CAN/Nup214 binds to both the cytoplasmic and the nucleoplasmic sides of the nuclear pore complex in overexpressing cells. Exp Cell Res. 1997;232:182–185. - PubMed
- Bogerd H P, Fridell R A, Madore S, Cullen B R. A novel cellular co-factor for HIV-1 Rev. Cell. 1995;82:485–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous