Persistent infection promotes cross-species transmissibility of mouse hepatitis virus - PubMed (original) (raw)
Persistent infection promotes cross-species transmissibility of mouse hepatitis virus
R S Baric et al. J Virol. 1999 Jan.
Abstract
Persistent infection with mouse hepatitis virus (MHV) strain A59 in murine DBT (delayed brain tumor) cells resulted in the emergence of host range variants, designated V51A and V51B, at 210 days postinfection. These host range mutants replicated efficiently in normally nonpermissive Chinese hamster ovary (CHO), in human hepatocarcinoma (HepG2), and to a lesser extent in human breast carcinoma (MCF7) cell lines. Little if any replication was noted in baby hamster kidney (BHK), green African monkey kidney (COS-7), feline kidney (CRFK), and swine testicular (ST) cell lines. By fluorescent antibody (FA) staining, persistent viruses V10B and V30B, isolated at days 38 and 119 days postinfection, also demonstrated very low levels of replication in human HepG2 cells. These data suggest that persistence may rapidly select for host range expansion of animal viruses. Pretreatment of HepG2 cells with a polyclonal antibody directed against human carcinoembryonic antigens (CEA) or with some monoclonal antibodies (Col-1, Col-4, Col-12, and Col-14) that bind human CEA significantly inhibited V51B infection. Under identical conditions, little or no blockade was evident with other monoclonal antibodies (kat4c or Col-6) which also bind the human CEA glycoproteins. In addition, an antibody (EDDA) directed against irrelevant antigens did not block V51B replication. Pretreatment with the Col-4 and Col-14 antibodies did not block Sindbis virus replication in HepG2 cells or MHV infection in DBT cells, suggesting that one or more CEA glycoproteins likely functioned as receptors for V51B entry into human cell lines. To test this hypothesis, the human biliary glycoprotein (Bgp) and CEA genes were cloned and expressed in normally nonpermissive BHK cell lines by using noncytopathic Sindbis virus replicons (pSinRep19). By growth curves and FA staining, human CEA and to a much lesser extent human Bgp functioned as receptors for V51B entry. Furthermore, V51B replication was blocked with polyclonal antiserum directed against human CEA and Bgp. Under identical conditions, the parental MHV strain A59 failed to replicate in BHK cells expressing human Bgp or CEA. These data suggest that MHV persistence may promote virus cross-species transmissibility by selecting for virus variants that recognize phylogenetic homologues of the normal receptor.
Figures
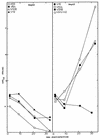
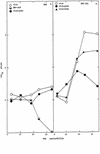
FIG. 1
Persistent virus replication in HepG2 cells. Cultures of HepG2 cells (105) were infected with V8A, V10B, V30B, V51A, V51B, MHV-A59, or the MHV-H2 host range variant at an MOI of 5 for 1 h at room temperature. Virus samples were harvested at the designated times and assayed by plaque assay.
FIG. 2
Persistent virus antigen expression in HepG2 cells. Cultures of HepG2 cell lines in eight-chamber LabTek chambers were infected with V51B (A), V10B (B), V30B (C), or MHV-A59 (D) at an MOI of 5 for 1 h. The cultures were stained with polyclonal MHV-A59 antiserum at 18 h postinfection.
FIG. 3
Blockade experiments in HepG2 cells. Cultures of HepG2 cells in eight-chamber LabTek slides were untreated (w/o) or treated with various polyclonal or monoclonal antibodies against hCEA genes at a 1:4 dilution for 1 h at room temperature. The antibodies were removed, and the cultures were infected with V51B at an MOI of 5 for 1 h. The virus inoculum was removed, and complete medium containing a 1:20 dilution of the same antibody was added to the chamber. Virus samples were taken at the indicated times, and virus titers were determined by plaque assay in DBT-9 cells.
FIG. 4
hCEA glycoprotein expression in HepG2 cells. HepG2 cells were pretreated with various antisera for 30 min at room temperature. After extensive washing, FITC-conjugated rabbit anti-mouse IgG antiserum was added for 30 min at room temperature. Following additional washing, the cells were analyzed by FACS techniques. (A) Col-1; (B) Col-12; (C) Col-14; (D) Col-6; (E) Kat4c.
FIG. 5
pSinRep19 replicons do not inhibit MHV infection. To determine if the noncytopathic pSinRep19 replicons block MHV replication, cultures of BHK-MHVR cells were transfected with the pSinRep19/T7pol transcripts and selected with puromycin (5 μg/ml) for several days. Cultures of BHK-MHVR/SinT7pol, BHK-MHVR, and BHK cells were infected with MHV-A59 at an MOI of 5 for 1 h. Virus samples were harvested at the indicated times and assayed by plaque assay in DBT cells.
FIG. 6
Expression of hCEA and hBgp in BHK cells. BHK cells were transfected with transcripts from the pSinRep19-hBgp and pSinRep19-hCEA replicons and selected with puromycin (5 μg/ml) for several days. By using monoclonal antibody Kat4c, the cultures were sorted into cell lines BHK-hBgp1 (A), BHK-hBgp1+ (B), and BHK-hCEA (C). These cell lines were grown into large stocks of cell lines expressing relatively uniform levels of hCEA or hBgp. FACS analysis was performed with monoclonal antibody Kat4c as previously described (12).
FIG. 6
Expression of hCEA and hBgp in BHK cells. BHK cells were transfected with transcripts from the pSinRep19-hBgp and pSinRep19-hCEA replicons and selected with puromycin (5 μg/ml) for several days. By using monoclonal antibody Kat4c, the cultures were sorted into cell lines BHK-hBgp1 (A), BHK-hBgp1+ (B), and BHK-hCEA (C). These cell lines were grown into large stocks of cell lines expressing relatively uniform levels of hCEA or hBgp. FACS analysis was performed with monoclonal antibody Kat4c as previously described (12).
FIG. 7
MHV replication in BHK cells expressing hCEA glycoproteins. Cultures of BHK, BHK-hCEA, BHK-hBgp1, and BHK-hBgp1+ cells were infected with V51B or MHV-A59, as indicated, at an MOI of 5 for 1 h at room temperature. In some experiments, the cells had been pretreated with a 1:4 dilution of pCEA or nonspecific antibody EDDA for 30 min prior to infection. Following infection, the cultures were washed extensively and virus were samples taken at the indicated times.
FIG. 7
MHV replication in BHK cells expressing hCEA glycoproteins. Cultures of BHK, BHK-hCEA, BHK-hBgp1, and BHK-hBgp1+ cells were infected with V51B or MHV-A59, as indicated, at an MOI of 5 for 1 h at room temperature. In some experiments, the cells had been pretreated with a 1:4 dilution of pCEA or nonspecific antibody EDDA for 30 min prior to infection. Following infection, the cultures were washed extensively and virus were samples taken at the indicated times.
FIG. 7
MHV replication in BHK cells expressing hCEA glycoproteins. Cultures of BHK, BHK-hCEA, BHK-hBgp1, and BHK-hBgp1+ cells were infected with V51B or MHV-A59, as indicated, at an MOI of 5 for 1 h at room temperature. In some experiments, the cells had been pretreated with a 1:4 dilution of pCEA or nonspecific antibody EDDA for 30 min prior to infection. Following infection, the cultures were washed extensively and virus were samples taken at the indicated times.
FIG. 8
Virus antigen in BHK cells expressing hCEA glycoproteins. Cultures treated as described for Fig. 7 were fixed and FA stained for the presence of viral antigen. (A) BHK-hCEA cells infected with V51B; (B) BHK cells infected with V51B; (C) BHK-hCEA cells infected with MHV-A59; (D) BHK cells infected with MHV-A59.
FIG. 9
Persistent virus replication in BHK-hCEA cells. Cultures of BHK-hCEA cells (106) were infected with MHV-A59, V10B, V30B, V51A, and V51B at an MOI of 5 for 1 h at room temperature. Virus titers were harvested at the designated times for analysis by plaque assay.
Similar articles
- Human biliary glycoproteins function as receptors for interspecies transfer of mouse hepatitis virus.
Hensley LE, Baric RS. Hensley LE, et al. Adv Exp Med Biol. 1998;440:43-52. doi: 10.1007/978-1-4615-5331-1_6. Adv Exp Med Biol. 1998. PMID: 9782263 - Virus-receptor interactions and interspecies transfer of a mouse hepatitis virus.
Hensley LE, Holmes KV, Beauchemin N, Baric RS. Hensley LE, et al. Adv Exp Med Biol. 1998;440:33-41. doi: 10.1007/978-1-4615-5331-1_5. Adv Exp Med Biol. 1998. PMID: 9782262 - The murine coronavirus mouse hepatitis virus strain A59 from persistently infected murine cells exhibits an extended host range.
Schickli JH, Zelus BD, Wentworth DE, Sawicki SG, Holmes KV. Schickli JH, et al. J Virol. 1997 Dec;71(12):9499-507. doi: 10.1128/JVI.71.12.9499-9507.1997. J Virol. 1997. PMID: 9371612 Free PMC article. - Characterization of a new gene that encodes a functional MHV receptor and progress in the identification of the virus-binding site(s).
Dveksler G, Nedellec P, Lu JH, Keck U, Basile A, Cardellichio C, Zimmermann W, Beauchemin N, Holmes KV. Dveksler G, et al. Adv Exp Med Biol. 1995;380:345-50. doi: 10.1007/978-1-4615-1899-0_56. Adv Exp Med Biol. 1995. PMID: 8830505 Review. - Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression.
Godfraind C, Coutelier JP. Godfraind C, et al. Histol Histopathol. 1998 Jan;13(1):181-99. doi: 10.14670/HH-13.181. Histol Histopathol. 1998. PMID: 9476648 Review.
Cited by
- A MERS-CoV antibody neutralizes a pre-emerging group 2c bat coronavirus.
Tse LV, Hou YJ, McFadden E, Lee RE, Scobey TD, Leist SR, Martinez DR, Meganck RM, Schäfer A, Yount BL, Mascenik T, Powers JM, Randell SH, Zhang Y, Wang L, Mascola J, McLellan JS, Baric RS. Tse LV, et al. Sci Transl Med. 2023 Sep 27;15(715):eadg5567. doi: 10.1126/scitranslmed.adg5567. Epub 2023 Sep 27. Sci Transl Med. 2023. PMID: 37756379 Free PMC article. - Evolution, Ecology, and Zoonotic Transmission of Betacoronaviruses: A Review.
Jelinek HF, Mousa M, Alefishat E, Osman W, Spence I, Bu D, Feng SF, Byrd J, Magni PA, Sahibzada S, Tay GK, Alsafar HS. Jelinek HF, et al. Front Vet Sci. 2021 May 20;8:644414. doi: 10.3389/fvets.2021.644414. eCollection 2021. Front Vet Sci. 2021. PMID: 34095271 Free PMC article. Review. - The V617I Substitution in Avian Coronavirus IBV Spike Protein Plays a Crucial Role in Adaptation to Primary Chicken Kidney Cells.
Jiang Y, Gao M, Cheng X, Yu Y, Shen X, Li J, Zhou S. Jiang Y, et al. Front Microbiol. 2020 Dec 18;11:604335. doi: 10.3389/fmicb.2020.604335. eCollection 2020. Front Microbiol. 2020. PMID: 33391226 Free PMC article. - Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes.
Guo H, Hu BJ, Yang XL, Zeng LP, Li B, Ouyang S, Shi ZL. Guo H, et al. J Virol. 2020 Sep 29;94(20):e00902-20. doi: 10.1128/JVI.00902-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32699095 Free PMC article. - Genetic diversity and ecology of coronaviruses hosted by cave-dwelling bats in Gabon.
Maganga GD, Pinto A, Mombo IM, Madjitobaye M, Mbeang Beyeme AM, Boundenga L, Ar Gouilh M, N'Dilimabaka N, Drexler JF, Drosten C, Leroy EM. Maganga GD, et al. Sci Rep. 2020 Apr 30;10(1):7314. doi: 10.1038/s41598-020-64159-1. Sci Rep. 2020. PMID: 32355260 Free PMC article.
References
- Ampel N M. Plagues—what’s past is present: thoughts on the origin and history of new infectious diseases. Rev Infect Dis. 1991;13:658–665. - PubMed
- Baric, R. S., et al. Unpublished data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous