Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain - PubMed (original) (raw)
Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain
J H Miner et al. J Cell Biol. 1998.
Abstract
Laminins are the major noncollagenous glycoproteins of all basal laminae (BLs). They are alpha/beta/gamma heterotrimers assembled from 10 known chains, and they subserve both structural and signaling roles. Previously described mutations in laminin chain genes result in diverse disorders that are manifested postnatally and therefore provide little insight into laminin's roles in embryonic development. Here, we show that the laminin alpha5 chain is required during embryogenesis. The alpha5 chain is present in virtually all BLs of early somite stage embryos and then becomes restricted to specific BLs as development proceeds, including those of the surface ectoderm and placental vasculature. BLs that lose alpha5 retain or acquire other alpha chains. Embryos lacking laminin alpha5 die late in embryogenesis. They exhibit multiple developmental defects, including failure of anterior neural tube closure (exencephaly), failure of digit septation (syndactyly), and dysmorphogenesis of the placental labyrinth. These defects are all attributable to defects in BLs that are alpha5 positive in controls and that appear ultrastructurally abnormal in its absence. Other laminin alpha chains accumulate in these BLs, but this compensation is apparently functionally inadequate. Our results identify new roles for laminins and BLs in diverse developmental processes.
Figures
Figure 2
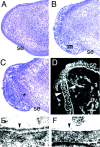
Targeted mutagenesis of the Lama5 gene. (A) The targeting vector deleted exons encoding 113 amino acids and replaced them with an in frame lacZ cDNA and the PGK neo selectable marker. N, NcoI; X, XbaI. (B) Southern analysis of genomic DNA from E13.5 embryos demonstrates the existence of the three expected genotypes, confirming that targeting was successful and that homozygous mutants were alive at this age. The probe, shown in A, was from outside the short arm of the targeting vector. (C and D) Presence of laminin α5 protein in control (C) but not mutant (D) distal limb ectodermal BL at E13.5, detected immunohistochemically with antisera to epitopes outside of the targeted region. Bar, 50 μm.
Figure 2
Targeted mutagenesis of the Lama5 gene. (A) The targeting vector deleted exons encoding 113 amino acids and replaced them with an in frame lacZ cDNA and the PGK neo selectable marker. N, NcoI; X, XbaI. (B) Southern analysis of genomic DNA from E13.5 embryos demonstrates the existence of the three expected genotypes, confirming that targeting was successful and that homozygous mutants were alive at this age. The probe, shown in A, was from outside the short arm of the targeting vector. (C and D) Presence of laminin α5 protein in control (C) but not mutant (D) distal limb ectodermal BL at E13.5, detected immunohistochemically with antisera to epitopes outside of the targeted region. Bar, 50 μm.
Figure 1
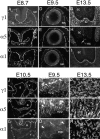
Distribution of laminin α1 and α5 chains in embryonic and extraembryonic BLs. Sections of embryos at the indicated ages were labeled with antibodies specific for the laminin α1, α5, or γ1 chains. The γ1 chain is present in, and thus serves to mark, all BLs. (A–C) BLs underlying the unclosed neuroepithelium (n), the surface ectoderm (se), and the gut epithelium (g) contain both α1 and α5 chains at E8.7. (D–F) After neural tube closure, α5 levels decrease in the neuroepithelial BL, and α1 levels decrease in the surface ectodermal BL. (G–I) By E13.5, α5 is confined to the BL adjacent to the floorplate of the spinal cord (sc) (arrow in H) and to the notochord (nc), whereas α1 is found throughout the pial BL but is absent from the notochord. Neither chain is present in BLs of blood vessels within and outside the spinal cord. These vascular BLs contain laminin α4 (not shown). (J–L) In E10.5 heart, both α1 and α5 are found in the atria (a) and in the ventricles (v), though levels of α1 are low in ventricle. (M–O) In the nascent placental labyrinth, the BLs at the base of embryonic blood vessels in the ectoplacental plate (epp) contain both α1 and α5, whereas the tips of vessels that have migrated towards the maternal blood spaces (arrowheads) contain α5 but lack α1. N and O are from a single, doubly labeled section. (P–R) In E13.5 placental labyrinth, the fetal blood vessel BLs contain both α1 and α5 throughout their lengths. Bar, 10 μm for A–C and P–R, 20 μm for all other panels.
Figure 3
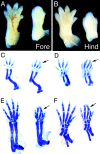
Syndactyly in Lama5 −/− embryos. Controls are on the left and mutants on the right. (A and B) Whole mount dorsal views of E14.5 fore- and hindlimbs. Digits 1 and 5 are partially septated in the mutant hindlimb. (C and D) Alcian blue staining at E13.5 demonstrates proper patterning in the mutant. Digits 2 and 3 are closely apposed but are not fused (arrows). (E and F) Alcian blue staining at E15.5 shows complete fusion of mutant digits 2 and 3 (arrows) and the absence of distal phalanges from digits 2–4 in the mutant forelimb.
Figure 4
Discontinuity of epidermis and BL in the distal limb. (A–C) Toluidine blue–stained semithin sections of distal limb from control (A) and mutant (B and C) E14.5 embryos. The surface ectoderm (se) is continuous in controls (A). In mutants, in contrast, the ectoderm has been breached, and extruded mesenchymal cells (m) have migrated along the outer surface of the limb (B). A nearby section from the same mutant limb shows a thickened surface ectoderm (*) between the outer and inner mesenchymal populations (C). (D) Immunostaining of Lama5 −/− limb with an antibody to laminin γ1 demonstrates the presence of BL material on both sides of the thickened ectoderm (arrowheads), suggesting that the ectoderm has maintained a proper relationship with the displaced mesenchymal cells. (E and F) Ultrastructural analysis of distal limb BL at E14.5 shows a dense, continuous BL (arrowhead) in the control (E) but a patchy, discontinuous BL in the mutant (F). Bars: (in D) 62.5 μm for A–C, 50 μm for D; (in F) 0.25 μm for E and F.
Figure 5
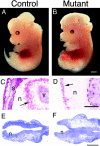
Exencephaly in Lama5 −/− embryos. (A and B) Whole mount views of control and mutant embryos at E13.5. Exencephalics lack the skin and skull that normally enclose the brain and have a topologically “inside out” brain that does not develop properly. The skin and skull of the control was dissected away to allow direct comparison of mutant and normal brains. (C and D) Immunohistochemical localization of proliferating cells with anti-BrdU and alkaline phosphatase chemistry after labeling for 1 h in utero with BrdU. (C) In normal neural tissue (n), BrdU-labeled cells (arrow) are confined to the ventricular zone. v, ventricle. (D) In exencephalics, labeled cells (arrow) are found on the outer surface of the neural tissue. This surface would have been ventricular had the neural tube closed, but now it is in direct contact with amniotic fluid. Skin and skull (s) overlay the brain in the control but are missing from the mutant. Pia is indicated by dashed lines. (E and F) Toluidine blue–stained sections through the anterior of E8.7 control and mutant embryos; the neural tube is unclosed at this stage in both control and mutant. Electron micrographs shown in Fig. 6 were obtained from these regions. Bars: (B) 1 mm; (D) 0.25 mm; (F) 0.2 mm.
Figure 6
Electron micrographs of epithelial BLs in the heads of control (B–E) and mutant (F–I) embryos at E8.7. (A) Drawing indicating the approximate origins of the sections shown in B–I. The narrow line represents the BL. (B and F) Lateral cranial surface ectoderm. (C and G) Surface ectoderm near the dorsal midline. (D and H) Junction of surface ectoderm and neuroepithelium. (E and I) Neuroepithelium. BLs are intact in both mutants and controls beneath lateral ectoderm and neuroepithelium. BLs are discontinuous in both mutants and controls near the junction of ectoderm and neuroepithelium. Beneath the surface ectoderm near the neural folds, however, control BL is intact, whereas mutant BL is disrupted. This region of the ectoderm has been shown to be important in neural tube closure (see text). n, neuroepithelium; se, surface ectoderm; arrowheads, BL. Bar, 0.5 μm.
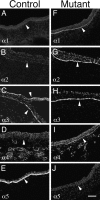
Figure 7
Molecular compensation for loss of laminin α5 in Lama5 −/− ectoderm. E13.5 controls (A–E) and mutants (F–J) were stained with antisera specific for the five known laminin α chains. Micrographs show surface ectoderm from the flank. Normal ectodermal BL (arrowheads) contained only α3 and α5 at this age, but mutant BL contained the α1–α4 chains. Thus, α1, α2, and α4 were upregulated in response to loss of α5. Bar, 50 μm.
Figure 8
Placental dysmorphogenesis in the absence of laminin α5. (A and B) Immunostaining for laminin γ1 at E13.5 reveals the network of fetal blood vessel BLs in the placental labyrinth. The mutant vessels (B) are fewer, less branched, and wider bore than those in the control (A). (C and D) Toluidine blue staining of E16.5 control (C) and mutant (D) labyrinth shows that large bore mutant vessels (arrows in D) are still present at this later stage. The endothelium appears to have detached from the trophoblasts in these vessels. (E and F) Transmission electron micrographs show definitively that some mutant fetal vessels (F) have become detached from the trophoblasts, leaving a cell-free space between the endothelium (e) and the trophoblasts (t), while in the control (E) the two cell layers remain closely apposed. (G and H) Higher power micrographs of vascular BL. In control (G), both endothelium and trophoblast are tightly adherent to this BL, whereas in the mutant (H), the BL is patchy and has lost attachment to the trophoblasts. Bars: (in D) 100 μm for A and B, 25 μm for C and D; (in F) 1 μm for E and F; (in H) 0.25 μm for G and H.
Similar articles
- Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice.
Patton BL, Miner JH, Chiu AY, Sanes JR. Patton BL, et al. J Cell Biol. 1997 Dec 15;139(6):1507-21. doi: 10.1083/jcb.139.6.1507. J Cell Biol. 1997. PMID: 9396756 Free PMC article. - Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function.
Kikkawa Y, Miner JH. Kikkawa Y, et al. Dev Biol. 2006 Aug 1;296(1):265-77. doi: 10.1016/j.ydbio.2006.04.463. Epub 2006 May 5. Dev Biol. 2006. PMID: 16750824 - The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform.
Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. Miner JH, et al. J Cell Biol. 1997 May 5;137(3):685-701. doi: 10.1083/jcb.137.3.685. J Cell Biol. 1997. PMID: 9151674 Free PMC article. - Expression and function of laminins in the embryonic and mature vasculature.
Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Hallmann R, et al. Physiol Rev. 2005 Jul;85(3):979-1000. doi: 10.1152/physrev.00014.2004. Physiol Rev. 2005. PMID: 15987800 Review. - Laminins during muscle development and in muscular dystrophies.
Gullberg D, Tiger CF, Velling T. Gullberg D, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):442-60. doi: 10.1007/pl00000616. Cell Mol Life Sci. 1999. PMID: 11212297 Free PMC article. Review.
Cited by
- Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization.
Stratman AN, Davis GE. Stratman AN, et al. Microsc Microanal. 2012 Feb;18(1):68-80. doi: 10.1017/S1431927611012402. Epub 2011 Dec 14. Microsc Microanal. 2012. PMID: 22166617 Free PMC article. Review. - Laminin is required to orient epithelial polarity in the C. elegans pharynx.
Rasmussen JP, Reddy SS, Priess JR. Rasmussen JP, et al. Development. 2012 Jun;139(11):2050-60. doi: 10.1242/dev.078360. Epub 2012 Apr 25. Development. 2012. PMID: 22535412 Free PMC article. - Laminin-10 is crucial for hair morphogenesis.
Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, Bradley M, Keene DR, Oro AE, Miner JH, Marinkovich MP. Li J, et al. EMBO J. 2003 May 15;22(10):2400-10. doi: 10.1093/emboj/cdg239. EMBO J. 2003. PMID: 12743034 Free PMC article. - Laminin isoforms in development and disease.
Schéele S, Nyström A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Schéele S, et al. J Mol Med (Berl). 2007 Aug;85(8):825-36. doi: 10.1007/s00109-007-0182-5. Epub 2007 Apr 11. J Mol Med (Berl). 2007. PMID: 17426950 Review. - Basal cell adhesion molecule promotes metastasis-associated processes in ovarian cancer.
Sivakumar S, Lieber S, Librizzi D, Keber C, Sommerfeld L, Finkernagel F, Roth K, Reinartz S, Bartsch JW, Graumann J, Müller-Brüsselbach S, Müller R. Sivakumar S, et al. Clin Transl Med. 2023 Jan;13(1):e1176. doi: 10.1002/ctm2.1176. Clin Transl Med. 2023. PMID: 36647260 Free PMC article.
References
- Aberdam D, Aguzzi A, Baudoin C, Galliano MF, Ortonne JP, Meneguzzi G. Developmental expression of nicein adhesion protein (laminin-5) subunits suggests multiple morphogenic roles. Cell Adhes Commun. 1994;2:115–129. - PubMed
- Abrahamson DR, Irwin MH, St. John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–3491. - PMC - PubMed
- Carter TC, Phillips RJS. Ragged, a semidominant coat texture mutant. J Hered. 1954;45:151–154.
- Cheng Y-S, Champliaud M-F, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272:31525–31532. - PubMed
- Chung AE, Jaffe R, Freeman IL, Vergnes JP, Braginsk JE, Carlin B. Properties of a basement membrane related glycoprotein synthesized by a mouse embryonal carcinoma-derived cell line. Cell. 1979;16:277–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases