Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons - PubMed (original) (raw)
Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons
Y X Li et al. J Neurosci. 1998.
Abstract
Brain-derived neurotrophic factor (BDNF), like other neurotrophins, has long-term effects on neuronal survival and differentiation; furthermore, recent work has shown that BDNF also can induce rapid changes in synaptic efficacy. We have investigated the mechanism(s) of these synaptic effects on cultured embryonic hippocampal neurons. In the presence of the GABAA receptor antagonist, picrotoxin, the application of BDNF (100 ng/ml) for 1-5 min increased the amplitude of evoked synaptic currents by 48 +/- 9% in 10 of 15 pairs of neurons and increased the frequency of EPSC bursts to 205 +/- 20% of the control levels. There was no detectable effect of BDNF on various measures of electrical excitability, including the resting membrane potential, input resistance, action potential threshold, and action potential amplitude. In addition, BDNF did not change the postsynaptic currents induced by the exogenous application of glutamate. BDNF did increase the frequency of miniature EPSCs (mEPSCs) (268.0 +/- 46.8% of control frequency), however, without affecting the mEPSC amplitude. The effect of BDNF on mEPSC frequency was blocked by the tyrosine kinase inhibitor K252a and also by the removal of extracellular calcium ([Ca2+]o). Fura-2 recordings showed that BDNF elicited an increase in intracellular calcium concentration ([Ca2+]c). This effect was dependent on [Ca2+]o; it was blocked by K252a and by thapsigargin, but not by caffeine. The results demonstrate that BDNF enhances glutamatergic synaptic transmission at a presynaptic locus and that this effect is accompanied by a rise in [Ca2+]c that requires the release of Ca2+ from IP3-gated stores.
Figures
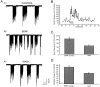
Fig. 1.
Bursts of synaptic activity in whole-cell recordings of E18 hippocampal neurons cultured after 2 weeks. The inward current deflections are produced by postsynaptic currents.A, Shown is an example of recordings. B, Shown is the time course of the BDNF effect. The effect of BDNF (100 ng/ml) on the burst frequency appeared ∼30 sec after application. The increased frequency and the increased magnitude of the integrated current per minute (denoted synaptic charge) recovered to baseline ∼5 min after washout of BDNF. C, D, Average values of bursting frequency and of synaptic charge in and after BDNF application.
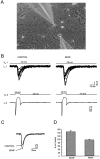
Fig. 2.
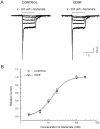
The effect of BDNF on evoked synaptic currents between paired neurons. A, Micrograph taken during dual whole-cell recordings was made from a pair of nearby cultured hippocampal neurons; note the images of the micropipette electrodes.B, The bottom panel shows the current in the presynaptic cell during a voltage step to −10 mV from a holding potential of −70 mV. The top panel superimposes 12 continuous EPSCs recorded simultaneously from the postsynaptic cell before and during BDNF application. C, The average of EPSCs recorded during 5 min before (CONTROL) and 2–5 min after the beginning of BDNF application. D, The average of the amplitude of evoked EPSCs during and after the application of BDNF.
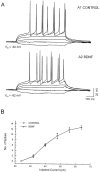
Fig. 3.
BDNF did not affect the electrical excitability of neurons. APV, CNQX, and picrotoxin were used to block the NMDA-, AMPA-, and GABA-induced responses from the input of other cells. Recordings were made in current-clamp mode. A, Shown are the I–V responses recorded from cells both before (A1) and during (A2) BDNF application. B, Shown is the relation between the amplitude of the injected current and the number of action potentials during the 600 msec current pulses.
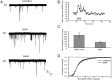
Fig. 4.
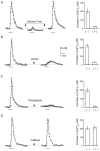
BDNF increased the frequency, but not the amplitude, distribution of mEPSCs. A, Shown are examples of recordings. TTX (100 n
m
) was present to block the release of transmitter caused by spontaneous action potentials.B, Shown is the time course of the BDNF effect. At ∼30 sec after BDNF application the frequency of mEPSCs was increased. The increased frequency recovered to the baseline value 5 min after the washout of BDNF. C, The average values of frequency during and after BDNF application. D, Averages of cumulative amplitude distributions of mEPSCs obtained before and during BDNF application (n = 9). There is no obvious difference (p > 0.05) between the distributions of amplitudes before and during BDNF application.
Fig. 5.
The increased frequency of mEPSCs caused by BDNF was blocked by K252a and was dependent on the extracellular calcium.
Fig. 6.
BDNF did not affect the sensitivity of glutamate receptors. A, Shown are inward currents induced by glutamate (2–200 μ
m
) before and during BDNF application.B, Shown is the dose–response relation for the glutamate-induced current, measured during the plateau (900 msec after the start of application; n = 5).
Fig. 7.
BDNF induced an increase in intracellular Ca2+. [Ca2+]c was measured by image ratio fluorescence microscopy with fura-2.A, A hippocampal cell responded to BDNF with an increase in [Ca2+]c. The BDNF-induced increase in [Ca2+]c disappeared in Ca2+-free solution and was restored after a switch to normal saline. B, K252a blocked the BDNF-induced increase in [Ca2+]c. C, Exposure to 2 μ
m
thapsigargin blocked the [Ca2+]c response. D, The [Ca2+]c response was present during exposure to caffeine-treated cells. (+) and (−) in the graphs indicate the BDNF-induced responses in the presence and absence of the drug, respectively. The bar in each panel indicates the period of BDNF application.
Similar articles
- Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons.
Mizoguchi Y, Monji A, Nabekura J. Mizoguchi Y, et al. Eur J Neurosci. 2002 Oct;16(8):1417-24. doi: 10.1046/j.1460-9568.2002.02198.x. Eur J Neurosci. 2002. PMID: 12405954 - Actions of brain-derived neurotrophic factor on evoked and spontaneous EPSCs dissociate with maturation of neurones cultured from rat visual cortex.
Taniguchi N, Takada N, Kimura F, Tsumoto T. Taniguchi N, et al. J Physiol. 2000 Sep 15;527 Pt 3(Pt 3):579-92. doi: 10.1111/j.1469-7793.2000.t01-1-00579.x. J Physiol. 2000. PMID: 10990542 Free PMC article. - Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal.
Iwata S, Wakita M, Shin MC, Fukuda A, Akaike N. Iwata S, et al. Brain Res Bull. 2013 Apr;93:39-46. doi: 10.1016/j.brainresbull.2012.11.002. Epub 2012 Nov 19. Brain Res Bull. 2013. PMID: 23174309 Review. - Journey of brain-derived neurotrophic factor: from intracellular trafficking to secretion.
Kojima M, Ishii C, Sano Y, Mizui T, Furuichi T. Kojima M, et al. Cell Tissue Res. 2020 Oct;382(1):125-134. doi: 10.1007/s00441-020-03274-x. Epub 2020 Sep 8. Cell Tissue Res. 2020. PMID: 32897423 Review.
Cited by
- What is the biological significance of BDNF mRNA targeting in the dendrites? Clues from epilepsy and cortical development.
Tongiorgi E, Domenici L, Simonato M. Tongiorgi E, et al. Mol Neurobiol. 2006 Feb;33(1):17-32. doi: 10.1385/MN:33:1:017. Mol Neurobiol. 2006. PMID: 16388108 Review. - BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses.
Tyler WJ, Pozzo-Miller LD. Tyler WJ, et al. J Neurosci. 2001 Jun 15;21(12):4249-58. doi: 10.1523/JNEUROSCI.21-12-04249.2001. J Neurosci. 2001. PMID: 11404410 Free PMC article. - Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF.
Bastioli G, Arnold JC, Mancini M, Mar AC, Gamallo-Lana B, Saadipour K, Chao MV, Rice ME. Bastioli G, et al. J Neurosci. 2022 Jun 8;42(23):4725-4736. doi: 10.1523/JNEUROSCI.2273-21.2022. Epub 2022 May 16. J Neurosci. 2022. PMID: 35577554 Free PMC article. - Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects.
Li Voti P, Conte A, Suppa A, Iezzi E, Bologna M, Aniello MS, Defazio G, Rothwell JC, Berardelli A. Li Voti P, et al. Exp Brain Res. 2011 Jul;212(1):91-9. doi: 10.1007/s00221-011-2700-5. Epub 2011 May 3. Exp Brain Res. 2011. PMID: 21537966 - Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons.
Prange O, Murphy TH. Prange O, et al. J Neurosci. 1999 Aug 1;19(15):6427-38. doi: 10.1523/JNEUROSCI.19-15-06427.1999. J Neurosci. 1999. PMID: 10414971 Free PMC article.
References
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. - PubMed
- Berg MM, Sternberg DW, Parada LF, Chao MV. K252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. - PubMed
- Berninger B, Poo M-M. Fast actions of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. - PubMed
- Berninger B, Garcia DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induced intracellular Ca2+ elevation in hippocampal neurons. NeuroReport. 1993;4:1303–1306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous