CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access - PubMed (original) (raw)
CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access
C von Gall et al. J Neurosci. 1998.
Abstract
The suprachiasmatic nucleus (SCN) is a central pacemaker in mammals, driving many endogenous circadian rhythms. An important pacemaker target is the regulation of a hormonal message for darkness, the circadian rhythm in melatonin synthesis. The endogenous clock within the SCN is synchronized to environmental light/dark cycles by photic information conveyed via the retinohypothalamic tract (RHT) and by the nocturnal melatonin signal that acts within a feedback loop. We investigated how melatonin intersects with the temporally gated resetting actions of two RHT transmitters, pituitary adenylate cyclase-activating polypeptide (PACAP) and glutamate. We analyzed immunocytochemically the inducible phosphorylation of the transcription factor Ca2+/cAMP response element-binding protein (CREB) in the SCN of a melatonin-proficient (C3H) and a melatonin-deficient (C57BL) mouse strain. In vivo, light-induced phase shifts in locomotor activity were consistently accompanied by CREB phosphorylation in the SCN of both strains. However, in the middle of subjective nighttime, light induced larger phase delays in C57BL than in C3H mice. In vitro, PACAP and glutamate induced CREB phosphorylation in the SCN of both mouse strains, with PACAP being more effective during late subjective daytime and glutamate being more effective during subjective nighttime. Melatonin suppressed PACAP- but not glutamate-induced phosphorylation of CREB. The distinct temporal domains during which glutamate and PACAP induce CREB phosphorylation imply that during the light/dark transition the SCN switches sensitivity between these two RHT transmitters. Because these temporal domains are not different between C3H and C57BL mice, the sensitivity windows are set independently of the rhythmic melatonin signal.
Figures
Fig. 1.
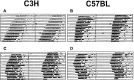
Light-induced effects on locomotor activity of C3H and C57BL mice. Animals were kept under constant darkness (dim red light). Representative double-plotted actograms of animal wheel-running activity are shown with asterisks indicating the time of light pulses (15 min; 1000 lux). Light presented during subjective day (CT06 and CT10) had little or no effect in C3H (A) and C57BL (B) mice, whereas light applied during subjective night (CT14 and CT18) resulted in stable phase delays in C3H (C) and C57BL (D) mice.
Fig. 2.
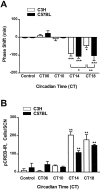
Comparison of light-induced effects on locomotor activity (A) and phosphorylation of CREB (B) in the mouse SCN. C3H and C57BL mice were kept in constant darkness (dim red light), and brief light pulses (15 min; 1000 lux) were delivered at the times indicated. Control animals were handled but not exposed to light. A, Light-induced phase shifts in locomotor activity were analyzed from recorded actograms. Negative values represent phase delays; positive values represent phase advances. Note that the light exposure at CT18 induced significantly smaller phase delays in C3H mice compared with C57BL mice. B, Light-induced pCREB-IR (see also Fig. 3) was quantified in cryostat-cut serial brain sections of the hypothalamic region containing the SCN. Each data point represents the mean ± SEM of 5–16 (A) or 3–9 (B) experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 3.
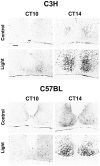
Immunocytochemical demonstration of a light-induced CREB phosphorylation in mouse SCN. Representative coronal sections through the hypothalamic region containing the SCN of C3H and C57BL mice are shown. Nuclear pCREB-IR in the SCN of mice under free-running conditions was induced when a brief light pulse (Light) was delivered 2 hr after activity onset (CT14) or in the middle of the subjective night (CT18; data not shown). Light stimulation had no effect when given 2 hr before activity onset (CT10) or in the middle of the subjective day (CT06; data not shown). Control animals not exposed to the light stimulus (Control) showed only a weak basal pCREB-IR within the SCN. Scale bar, 50 μm. oc, Optic chiasm.
Fig. 4.
Distribution of pCREB-IR and VIP-IR in the mouse SCN. C3H (left) and C57BL (right) mice, kept under standard light/dark conditions, were exposed to bright white light (10 min) at ZT14. Double-label immunocytochemistry using a confocal laser-scanning microscope shows the spatial distribution of light-induced pCREB-IR nuclei (red) and VIP-IR cells (green). Several cells show both nuclear pCREB-IR and cytoplasmic VIP-IR (arrows); some cells show either a nuclear pCREB-IR (arrowheads) or a cytoplasmic VIP-IR (asterisks) only. Scale bars: upper, 50 μm; lower, 25 μm. oc, Optic chiasm;3V, third ventricle.
Fig. 5.
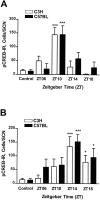
Semiquantitative analysis of the pCREB-IR induced by PACAP (A) or glutamate (B) in mouse SCN brain slices. A, PACAP application (100 n
m
) evoked a robust pCREB-IR in the SCN of C3H and C57BL mice when applied at ZT10 but had no effect at ZT14, ZT18, or ZT06. B, Glutamate application (100 μ
m
) induced pCREB-IR in the SCN of C3H and C57BL mice at ZT14 and ZT18 but had only slight effects at ZT06 and ZT10. Untreated slices (controls) showed a very low basal pCREB-IR (see also Fig. 7). Each data point represents the mean ± SEM of four to nine animals. Asterisks indicate significantly different values of stimulated slices compared with controls; *p < 0.05; ***p < 0.001.
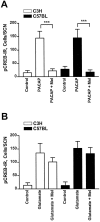
Fig. 6.
Semiquantitative analysis of the effects of melatonin on the PACAP- or the glutamate-induced pCREB-IR.A, A preceding incubation of SCN brain slices with melatonin (Mel; 1 n
m
) prevented the induction of a pCREB-IR by PACAP at ZT10 in both mouse strains.B, The glutamate-induced pCREB-IR in the SCN of both mouse strains at ZT14 was unaltered when slices were preincubated with melatonin. Each data point represents the mean ± SEM of four to eight animals; ***p < 0.001.
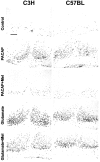
Fig. 7.
Effects of melatonin on the PACAP- or the glutamate-induced pCREB-IR. Untreated control slices (Control) showed no basal pCREB-IR in the SCN of both mouse strains after 2 hr in culture (ZT10) or after 6 hr in culture (ZT14; data not shown). PACAP application (PACAP) at ZT10 induced a nuclear pCREB-IR in the SCN of C3H and C57BL mice. The PACAP effect was suppressed when slices were preincubated with melatonin (PACAP + Mel). Glutamatergic stimulation (Glutamate) of slices at ZT14 evoked a nuclear pCREB-IR in the SCN of both mouse strains. Glutamate-effects were not affected by melatonin (Glutamate + Mel). In both mouse strains melatonin alone did not induce a pCREB-IR in the SCN (data not shown). Scale bar, 50 μm.oc, Optic chiasm.
Similar articles
- Melatonin limits transcriptional impact of phosphoCREB in the mouse SCN via the Mel1a receptor.
von Gall C, Weaver DR, Kock M, Korf HW, Stehle JH. von Gall C, et al. Neuroreport. 2000 Jun 26;11(9):1803-7. doi: 10.1097/00001756-200006260-00002. Neuroreport. 2000. PMID: 10884023 - An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.
Harmar AJ. Harmar AJ. J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review. - Roles of PACAP-containing retinal ganglion cells in circadian timing.
Hannibal J. Hannibal J. Int Rev Cytol. 2006;251:1-39. doi: 10.1016/S0074-7696(06)51001-0. Int Rev Cytol. 2006. PMID: 16939776 Review.
Cited by
- Neurogenetic basis for circadian regulation of metabolism by the hypothalamus.
Cedernaes J, Waldeck N, Bass J. Cedernaes J, et al. Genes Dev. 2019 Sep 1;33(17-18):1136-1158. doi: 10.1101/gad.328633.119. Genes Dev. 2019. PMID: 31481537 Free PMC article. Review. - Clock genes and sleep.
Landgraf D, Shostak A, Oster H. Landgraf D, et al. Pflugers Arch. 2012 Jan;463(1):3-14. doi: 10.1007/s00424-011-1003-9. Epub 2011 Aug 11. Pflugers Arch. 2012. PMID: 21833490 Review. - Melatonin's role in the timing of sleep onset is conserved in nocturnal mice.
Kim P, Garner N, Tatkovic A, Parsons R, Chunduri P, Vukovic J, Piper M, Pfeffer M, Weiergräber M, Oster H, Rawashdeh O. Kim P, et al. NPJ Biol Timing Sleep. 2024;1(1):13. doi: 10.1038/s44323-024-00013-1. Epub 2024 Nov 1. NPJ Biol Timing Sleep. 2024. PMID: 39493889 Free PMC article. - Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice.
Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Tahara Y, Shibata S. Sasaki H, et al. Sci Rep. 2016 Jun 8;6:27607. doi: 10.1038/srep27607. Sci Rep. 2016. PMID: 27271267 Free PMC article. - Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock.
Duffield GE, Robles-Murguia M, Hou TY, McDonald KA. Duffield GE, et al. Int J Mol Sci. 2021 Sep 6;22(17):9632. doi: 10.3390/ijms22179632. Int J Mol Sci. 2021. PMID: 34502541 Free PMC article.
References
- Armstrong SM, Cassone VM, Chesworth MJ, Redman JR, Short RV. Synchronization of mammalian circadian rhythms by melatonin. J Neural Transm. 1986;21:375–394. - PubMed
- Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–125. - PubMed
- Cassone VM, Roberts MH, Moore RY. Melatonin inhibits metabolic activity in the rat suprachiasmatic nuclei. Neurosci Lett. 1987;81:29–34. - PubMed
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous