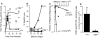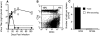Viral immune evasion due to persistence of activated T cells without effector function - PubMed (original) (raw)
Viral immune evasion due to persistence of activated T cells without effector function
A J Zajac et al. J Exp Med. 1998.
Abstract
We examined the regulation of virus-specific CD8 T cell responses during chronic lymphocytic choriomeningitis virus (LCMV) infection of mice. Our study shows that within the same persistently infected host, different mechanisms can operate to silence antiviral T cell responses; CD8 T cells specific to one dominant viral epitope were deleted, whereas CD8 T cells responding to another dominant epitope persisted indefinitely. These virus-specific CD8 T cells expressed activation markers (CD69(hi), CD44(hi), CD62Llo) and proliferated in vivo but were unable to elaborate any antiviral effector functions. This unresponsive phenotype was more pronounced under conditions of CD4 T cell deficiency, highlighting the importance of CD8- CD4 T cell collaboration in controlling persistent infections. Importantly, in the presence of CD4 T cell help, adequate CD8 effector activity was maintained and the chronic viral infection eventually resolved. The persistence of activated virus-specific CD8 T cells without effector function reveals a novel mechanism for silencing antiviral immune responses and also offers new possibilities for enhancing CD8 T cell immunity in chronically infected hosts.
Figures
Figure 1
CD8 T cell effector functions are lost during chronic infection of CD4−/− mice. (A) Serum virus titers were measured in +/+ (•) and age-matched CD4−/− (○) mice at various times after infection with LCMV-t1b. The limit of detection is indicated by the dashed line. (B) CTL activity of splenocytes from +/+ (▪, □) or CD4−/− (♦, ⋄) mice at 90 d after infection with LCMV-t1b was measured after 5 d restimulation in vitro. Target cells were either uninfected (open symbols) or LCMV infected (filled symbols). (C) Limiting dilution analysis was performed using splenocytes from either +/+ (▴) or CD4−/− (▵) mice at 90 d after infection with LCMV-t1b. The frequency of LCMV-specific cells per spleen is indicated in parentheses. (D) Virus-specific IFN-γ secreting cells were enumerated using single cell ELISPOT assays. Splenocytes were isolated from either +/+ or CD4−/− mice at 90 d after inoculation with LCMV-t1b.
Figure 2
T cell deletion and functional unresponsiveness are distinct mechanisms for silencing antiviral immune responses. Naive +/+ mice, +/+ immune mice (161 d after LCMV Armstrong infection; 2 × 105 PFU, i.p.), and +/+ or CD4−/− mice infected with LCMV-t1b (2 × 106 PFU, i.v.) 60 d previously were checked for the physical presence and functional responsiveness of LCMV-specific CD8 T cells. (A–H) LCMV-specific CD8 T cells were visualized using MHC class I tetramers complexed to viral peptides. Cells were costained with anti-CD8–PE, anti-CD44–FITC, and either Db(GP33–41) or Db(NP396–404) tetramers conjugated to allophycocyanin. The histograms show gated CD8 lymphocytes, and the percentage of CD8 cells costaining with either Db(GP33–41) or Db(NP396–404) are indicated in the corresponding upper right quadrant. Where not shown values are <0.2%. (I–L) Numbers of LCMV-specific IFN-γ producing cells were enumerated using single-cell cytokine ELISPOT assays. The number of splenocytes producing IFN-γ after stimulation with either GP33–41 (black bars) or NP396–404 peptides (hatched bars) are shown (± SD).
Figure 3
Kinetics of LCMV-specific CD8 T cell responses during chronic infection. Splenocytes were prepared from either +/+ or CD4−/− mice at various days after infection with LCMV-clone 13 (2 × 106 PFU, i.v.). The total numbers of GP33- (□) and NP396- (○) specific CD8 T cells were enumerated by staining with recombinant MHC H-2Db tetramers, and IFN-γ ELISPOT assays were performed to determine the functional responsiveness of these cells (GP33–41, ▪; NP396–404, •). Serum virus titers were also determined and are represented by the gray area. Mean values are shown for two to four mice at each time point and the limit of detection is represented by the dashed line. Standard deviations were <10%.
Figure 4
Chronic LCMV infection and CD8 T cell unresponsiveness ensue after transient loss of CD4 T cells. +/+ mice were transiently depleted of CD4 T cells by injection with the anti-CD4 antibody, GK1.5, 1 d before and 3 d after infection with LCMV-t1b. (A) Serum virus titers were determined in +/+ (•) and GK1.5 treated (□) +/+ mice at various days after infection. (B) Reconstitution of CD4 T cells in GK1.5-treated mice was determined at 60 d after infection with LCMV-t1b. Splenocytes were costained for CD4 and the activation marker CD44. The percentage of CD4+ cells are indicated in the upper quadrants. By this time point, both +/+ and GK1.5-treated mice contained comparable numbers of CD4 T cells. (C) Splenocytes from GK1.5-treated mice were prepared at 60 d after infection with LCMV-t1b and both the total number (measured by tetramer staining) and the number of IFN-γ–secreting (measured by ELISPOT) GP33- or NP396-specific CD8 T cells were determined. Greater than 98.5% of GP33-specific T cells were unresponsive and NP396-specific T cells were not detectable. In A and C the limit of detection is indicated by the dashed line. At least three mice were analyzed at each time point.
Figure 5
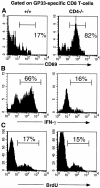
GP33-specific CD8 T cells in chronically infected CD4−/− mice are activated and proliferate, but are refractive to stimulation with PMA and ionomycin. (A) CD69 expression on freshly explanted splenocytes from +/+ and CD4−/− mice at 78 d after infection was assessed using flow cytometry. (B) IFN-γ production by GP33-specific CD8 T cells after PMA and ionomycin stimulation. Splenocytes were isolated from +/+ and CD4−/− mice at 108 d after infection with LCMV-t1b. (C) The in vivo proliferation of GP33-specific CD8 T cells in LCMV-t1b infected +/+ and CD4−/− mice (day 100 after infection) was assessed by in vivo BrdU labeling. Mice were fed BrdU in their drinking water for a total of 8 d. All histograms show Db(GP33–41)-positive cells and the percentage of GP33-specific T cells within each region is indicated.
Comment in
- The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses.
Kalams SA, Walker BD. Kalams SA, et al. J Exp Med. 1998 Dec 21;188(12):2199-204. doi: 10.1084/jem.188.12.2199. J Exp Med. 1998. PMID: 9858506 Free PMC article. Review. No abstract available.
Similar articles
- CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection.
Matloubian M, Concepcion RJ, Ahmed R. Matloubian M, et al. J Virol. 1994 Dec;68(12):8056-63. doi: 10.1128/JVI.68.12.8056-8063.1994. J Virol. 1994. PMID: 7966595 Free PMC article. - Ablation of CD8 and CD4 T cell responses by high viral loads.
Fuller MJ, Zajac AJ. Fuller MJ, et al. J Immunol. 2003 Jan 1;170(1):477-86. doi: 10.4049/jimmunol.170.1.477. J Immunol. 2003. PMID: 12496434 - Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections.
Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Fuller MJ, et al. J Immunol. 2004 Apr 1;172(7):4204-14. doi: 10.4049/jimmunol.172.7.4204. J Immunol. 2004. PMID: 15034033 - Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection.
Suresh M, Gao X, Fischer C, Miller NE, Tewari K. Suresh M, et al. J Virol. 2004 Apr;78(8):3906-18. doi: 10.1128/jvi.78.8.3906-3918.2004. J Virol. 2004. PMID: 15047807 Free PMC article. - Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.
Studstill CJ, Hahm B. Studstill CJ, et al. Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review.
Cited by
- Cold and hot tumors: from molecular mechanisms to targeted therapy.
Wu B, Zhang B, Li B, Wu H, Jiang M. Wu B, et al. Signal Transduct Target Ther. 2024 Oct 18;9(1):274. doi: 10.1038/s41392-024-01979-x. Signal Transduct Target Ther. 2024. PMID: 39420203 Free PMC article. Review. - Lymphocyte activation gene 3 served as a potential prognostic and immunological biomarker across various cancer types: a clinical and pan-cancer analysis.
Liu Y, Yao Y, Yang X, Wei M, Lu B, Dong K, Lyu D, Li Y, Guan W, Huang R, Xu G, Pan X. Liu Y, et al. Clin Transl Immunology. 2024 Oct 4;13(10):e70009. doi: 10.1002/cti2.70009. eCollection 2024. Clin Transl Immunology. 2024. PMID: 39372371 Free PMC article. - NitraTh epitope-based neoantigen vaccines for effective tumor immunotherapy.
Zhang W, Shi X, Huang S, Yu Q, Wu Z, Xie W, Li B, Xu Y, Gao Z, Li G, Qian Q, He T, Zheng J, Zhang T, Tong Y, Deng D, Gao X, Tian H, Yao W. Zhang W, et al. Cancer Immunol Immunother. 2024 Oct 3;73(12):245. doi: 10.1007/s00262-024-03830-2. Cancer Immunol Immunother. 2024. PMID: 39358493 Free PMC article. - Epigenetic tuning of PD-1 expression improves exhausted T cell function and viral control.
Weiss SA, Huang AY, Fung ME, Martinez D, Chen ACY, LaSalle TJ, Miller BC, Scharer CD, Hegde M, Nguyen TH, Rowe JH, Osborn JF, Patterson DG, Sifnugel N, Mei-An Nolan C, Davidson RA, Schwartz MA, Bally APR, Neeld DK, LaFleur MW, Boss JM, Doench JG, Nicholas Haining W, Sharpe AH, Sen DR. Weiss SA, et al. Nat Immunol. 2024 Oct;25(10):1871-1883. doi: 10.1038/s41590-024-01961-3. Epub 2024 Sep 17. Nat Immunol. 2024. PMID: 39289557 - Harnessing IL-2 for immunotherapy against cancer and chronic infection: a historical perspective and emerging trends.
Im SJ, Lee K, Ha SJ. Im SJ, et al. Exp Mol Med. 2024 Sep;56(9):1900-1908. doi: 10.1038/s12276-024-01301-3. Epub 2024 Sep 2. Exp Mol Med. 2024. PMID: 39218982 Free PMC article. Review.
References
- Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. - PubMed
- Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MBA. An essential role for type 1 interferon-γ in terminating persistent viral infection. Virology. 1995;212:244–250. - PubMed
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
- Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous