A potential role for the nuclear factor of activated T cells family of transcriptional regulatory proteins in adipogenesis - PubMed (original) (raw)
A potential role for the nuclear factor of activated T cells family of transcriptional regulatory proteins in adipogenesis
I C Ho et al. Proc Natl Acad Sci U S A. 1998.
Abstract
NFAT (nuclear factor of activated T cells) is a family of transcription factors implicated in the control of cytokine and early immune response gene expression. Recent studies have pointed to a role for NFAT proteins in gene regulation outside of the immune system. Herein we demonstrate that NFAT proteins are present in 3T3-L1 adipocytes and, upon fat cell differentiation, bind to and transactivate the promoter of the adipocyte-specific gene aP2. Further, fat cell differentiation is inhibited by cyclosporin A, a drug shown to prevent NFAT nuclear localization and hence function. Thus, these data suggest a role for NFAT transcription factors in the regulation of the aP2 gene and in the process of adipocyte differentiation.
Figures
Figure 1
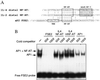
FSE2 element binds NFAT proteins. (A) The 5′ upstream promoter region of the aP2 gene with NFAT and AP-1 binding sites is highlighted and nucleotide sequence comparisons of NFAT/AP-1 binding sites in the aP2, IL-4, and IL-2 promoters are shown. (B) EMSA using T lymphocyte nuclear extracts and radiolabeled probe containing the FSE2 element (positions −127 to −101).
Figure 2
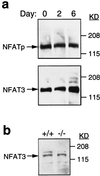
NFAT/AP-1 proteins are present in differentiated adipocytes and bind the FSE2. (a). Western blot analysis of cell extracts from differentiated 3T3L1 cells with antibodies to NFATp and NFAT3 is shown. (b) Western blot analysis of extracts prepared from the mesenteric fat of NFATp −/− and control NFAT +/+ mice with antibodies to NFAT3 is shown.
Figure 3
NFATp binds to FSE2 DNA only in differentiated adipocytes. EMSA and supershift experiments were performed with nuclear extracts from either undifferentiated (lane 5) or day 4 differentiated (the remaining lanes) 3T3-L1 cells, an FSE2 radiolabeled probe, and antibodies to NFATp and AP-1 proteins.
Figure 4
NFATp transactivates the aP2 promoter in adipocytes. (A) 3T3L1 cells were cotransfected with 20 μg of an aP2-CAT reporter (positions −168 to +20) and 20 μg of an NFATp expression plasmid (pRep4 NFATp) or control pRep4 plasmid in the presence or absence of phorbol 12-myristate 13-acetate (2.5 nM) and ionomycin (2 μM). CAT activity determined 48 hr later. (B) The experiment in A was repeated with either the wild-type aP2-CAT reporter or an aP2 reporter with a mutation in the NFAT site. The data were normalized to the activity of the pRep control vector (=1). One representative experiment of three is shown.
Figure 5
CsA inhibits adipocyte differentiation. (a) Undifferentiated 3T3-L1 cells. (b_–_d) T3L1 cells were differentiated for 6 days in the absence of control solvent (b), the presence of control solvent (c), or in CsA (10 μg/ml) (d) and stained with red oil O. The 3T3-L1 cells were differentiated in the presence of CsA (10 μg/ml) added at day 0 (d), day 1 (e), day 2 (f), day 3 (g), or day 4 (h).
Similar articles
- Distinct Ras effector pathways are involved in Fc epsilon R1 regulation of the transcriptional activity of Elk-1 and NFAT in mast cells.
Turner H, Cantrell DA. Turner H, et al. J Exp Med. 1997 Jan 6;185(1):43-53. doi: 10.1084/jem.185.1.43. J Exp Med. 1997. PMID: 8996240 Free PMC article. - Identification of a calcium-inducible, cyclosporine sensitive element in the IFN-gamma promoter that is a potential NFAT binding site.
Campbell PM, Pimm J, Ramassar V, Halloran PF. Campbell PM, et al. Transplantation. 1996 Mar 27;61(6):933-9. doi: 10.1097/00007890-199603270-00016. Transplantation. 1996. PMID: 8623163 - Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes.
Luo C, Burgeon E, Carew JA, McCaffrey PG, Badalian TM, Lane WS, Hogan PG, Rao A. Luo C, et al. Mol Cell Biol. 1996 Jul;16(7):3955-66. doi: 10.1128/MCB.16.7.3955. Mol Cell Biol. 1996. PMID: 8668213 Free PMC article. - Role of nuclear factor of activated T cells (NFAT) in the expression of interleukin-5 and other cytokines involved in the regulation of hemopoetic cells.
De Boer ML, Mordvinov VA, Thomas MA, Sanderson CJ. De Boer ML, et al. Int J Biochem Cell Biol. 1999 Oct;31(10):1221-36. doi: 10.1016/s1357-2725(99)00069-2. Int J Biochem Cell Biol. 1999. PMID: 10582349 Review. - Molecular regulation of adipocyte differentiation.
Cowherd RM, Lyle RE, McGehee RE Jr. Cowherd RM, et al. Semin Cell Dev Biol. 1999 Feb;10(1):3-10. doi: 10.1006/scdb.1998.0276. Semin Cell Dev Biol. 1999. PMID: 10355023 Review.
Cited by
- Expression, localisation and functional activation of NFAT-2 in normal human skin, psoriasis, and cultured keratocytes.
Al-Daraji WI, Malak TT, Prescott RJ, Abdellaoui A, Ali MM, Dabash T, Zelger BG, Zelger B. Al-Daraji WI, et al. Int J Clin Exp Med. 2009 Jun 18;2(2):176-92. Int J Clin Exp Med. 2009. PMID: 19684889 Free PMC article. - The interleukin-1 receptor associated kinase 1 contributes to the regulation of NFAT.
Wang D, Fasciano S, Li L. Wang D, et al. Mol Immunol. 2008 Sep;45(15):3902-8. doi: 10.1016/j.molimm.2008.06.023. Epub 2008 Aug 8. Mol Immunol. 2008. PMID: 18691762 Free PMC article. - Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency.
Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. Eto H, et al. Stem Cells Dev. 2013 Mar 15;22(6):985-97. doi: 10.1089/scd.2012.0442. Epub 2012 Dec 21. Stem Cells Dev. 2013. PMID: 23137270 Free PMC article. - Molecular mechanisms of adipocyte differentiation.
Tong Q, Hotamisligil GS. Tong Q, et al. Rev Endocr Metab Disord. 2001 Oct;2(4):349-55. doi: 10.1023/a:1011863414321. Rev Endocr Metab Disord. 2001. PMID: 11725721 Review. No abstract available. - NFATc1 regulation of TRAIL expression in human intestinal cells.
Wang Q, Zhou Y, Weiss HL, Chow CW, Evers BM. Wang Q, et al. PLoS One. 2011;6(5):e19882. doi: 10.1371/journal.pone.0019882. Epub 2011 May 16. PLoS One. 2011. PMID: 21603612 Free PMC article.
References
- Rao A, Luo C, Hogan P G. Annu Rev Immunol. 1997;15:707–747. - PubMed
- Shaw J, Utz P, Durand D, Toole J, Emmel E, Crabtree G. Science. 1988;241:202–205. - PubMed
- Crabtree G. Science. 1989;249:355–360. - PubMed
- Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. Nature (London) 1994;369:497–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials