Origin of a chloroplast protein importer - PubMed (original) (raw)
Origin of a chloroplast protein importer
B Bölter et al. Proc Natl Acad Sci U S A. 1998.
Abstract
During evolution, chloroplasts have relinquished the majority of their genes to the nucleus. The products of transferred genes are imported into the organelle with the help of an import machinery that is distributed across the inner and outer plastid membranes. The evolutionary origin of this machinery is puzzling because, in the putative predecessors, the cyanobacteria, the outer two membranes, the plasma membrane, and the lipopolysaccharide layer lack a functionally similar protein import system. A 75-kDa protein-conducting channel in the outer envelope of pea chloroplasts, Toc75, shares approximately 22% amino acid identity to a similarly sized protein, designated SynToc75, encoded in the Synechocystis PCC6803 genome. Here we show that SynToc75 is located in the outer membrane (lipopolysaccharide layer) of Synechocystis PCC6803 and that SynToc75 forms a voltage-gated, high conductance channel with a high affinity for polyamines and peptides in reconstituted liposomes. These findings suggest that a component of the chloroplast protein import system, Toc75, was recruited from a preexisting channel-forming protein of the cyanobacterial outer membrane. Furthermore, the presence of a protein in the chloroplastic outer envelope homologous to a cyanobacterial protein provides support for the prokaryotic nature of this chloroplastic membrane.
Figures
Figure 1
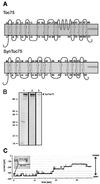
Secondary structure prediction and function of SynToc75. (A) Toc75 from pea and SynToc75 are predicted to form multiple transmembrane β-sheets (13). (B) Heterologously expressed SynToc75 was recovered from inclusion bodies (lane 1) and was purified further by anion exchange chromatography (lane 2). A Coomassie brilliant blue-stained SDS/PAGE is shown. In lanes 3 is an immunoblot of purified SynToc75. Lanes: 1 and 2, 10 μg of protein; 3, 1 μg of protein. (C) Current trace from a bilayer containing eight active SynToc75 channels after a voltage step from 0 to −80 mV with 250 mM KCl, 10 mM CaCl2, and 10 mM Hepes⋅Tris (pH 7.2) on both sides of the membrane.
Figure 2
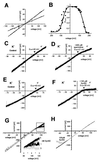
SynToc75 forms a high conductance cation-selective channel. (A) Current–voltage relationship of the fully open single channel (squares) and the most frequent subconductance level (circles) with 250 mM KCl on both sides of the membrane (data points were averaged from five independent bilayers, with SEMs <3.5%). (B) Voltage dependence of the probability for the SynToc75 channel being in any of its open states. To approach equilibrium of channel gating with respect to the applied membrane, voltages were applied for 5 min, but only the current recordings of the last minute were used to calculate open probability from the amplitude histograms (averages of at least three independent bilayers, with SEMs <6% of the values). (C and D) Influence of SynB2 (MLSRQQSQRQSQQSQRQSRYLL, _M_r = 2, 9) and spermidine on the SynToc75 conductivity. Voltage ramps (ΔV = 10 mV/s) were applied across bilayers containing multiple copies of the active SynToc75 channel. (C) Recording from a bilayer containing four active copies of the SynToc75 channel in asymmetrical 250 mM/20 mM KCl, 10 mM Mops/Tris (pH 7.0) buffer (cis/trans), control. (D) Same bilayer as in C, but after addition of 400 μM spermidine to the trans compartment. (E) Recording from a bilayer containing four active copies of the SynToc75 channel in asymmetrical 250 mM/20 mM KCl, 10 mM Mops/Tris (pH 7.0) buffer (cis/trans), control. (F) Same bilayer as in E, but after addition of 100 μM SynB2 to the trans compartment. (G) Recording from a bilayer containing 12 active copies of the SynToc75 channel in asymmetrical 250 mM/20 mM KCl, 10 mM Mops/Tris (pH 7.0) buffer (cis/trans). Black, control; gray/light gray, after addition of 1/5 nM SynB2 respectively to the trans compartment. (H) Recording from a bilayer containing 44 active copies of the SynToc75 channel in asymmetrical 250 mM/20 mM KCl, 10 mM Mops/Tris (pH 7.0) buffer (cis/trans). Black, control; gray, after addition of 100 μM SynB2 to the cis compartment.
Figure 3
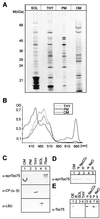
Localization of SynToc75 in cellular subfractions of Synechocystis PCC6803. (A) The polypeptide composition of soluble proteins (SOL), thylakoids (THY), plasma membrane (PM), and outer membrane (OM) was analyzed by PAGE and silver-staining. (B) Adsorption spectra were recorded from cyanobacterial membranes extracted by 80% (vol/vol) diethyl ketone. (C) Immunoblot analysis using antisera (α) against SynToc75, ATPase α- and β- subunit from spinach (CF1 α/β), Rubisco (LSU), and heterologously expressed SynToc75 (75ex). (D) Synechocystis outer membranes (lane 1) were extracted with 0.5 M Na2CO3 (pH 11.5) (lanes 2, 3) or 1 M NaCl (lanes 4, 5) and were separated into an insoluble (P) and a soluble (S) protein fraction. An immunoblot is shown. (E) Localization of Toc75 in pea chloroplast outer envelope (OE, lane 1), inner envelope (IE, lane 2), stromal proteins (SOL, lane 3), and thylakoids (THY, lane 4). Outer envelope membranes were treated with Na2CO3 or NaCl and fractionated as in D. An immunoblot is shown using an antiserum raised against peaToc75.
Figure 4
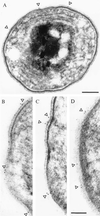
Immunogold labeling of ultra-thin sections of Synechocystis PCC6803 by using αSynToc75. Cells were fixed with 0.5% paraformaldehyd and were embedded in LRWhite by using standard methods. Colloidal gold ( 5 nm) coupled to the secondary antibody was used for visualization. The position of gold grains is indicated by arrowheads. [Bars = 200 nm (A), and 100 nm (B_–_D).] (A, ×70,000; B_–_D, ×125,000.)
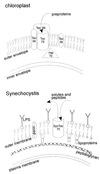
Figure 5
Working model of the localization and origin of a protein importer in cyanobacteria and pea chloroplasts.
Similar articles
- The evolutionary origin of the protein-translocating channel of chloroplastic envelope membranes: identification of a cyanobacterial homolog.
Reumann S, Davila-Aponte J, Keegstra K. Reumann S, et al. Proc Natl Acad Sci U S A. 1999 Jan 19;96(2):784-9. doi: 10.1073/pnas.96.2.784. Proc Natl Acad Sci U S A. 1999. PMID: 9892711 Free PMC article. - A Toc75-like protein import channel is abundant in chloroplasts.
Eckart K, Eichacker L, Sohrt K, Schleiff E, Heins L, Soll J. Eckart K, et al. EMBO Rep. 2002 Jun;3(6):557-62. doi: 10.1093/embo-reports/kvf110. Epub 2002 May 24. EMBO Rep. 2002. PMID: 12034753 Free PMC article. - Reconstitution of a chloroplast protein import channel.
Hinnah SC, Hill K, Wagner R, Schlicher T, Soll J. Hinnah SC, et al. EMBO J. 1997 Dec 15;16(24):7351-60. doi: 10.1093/emboj/16.24.7351. EMBO J. 1997. PMID: 9405364 Free PMC article. - Mechanism of protein import across the chloroplast envelope.
Chen K, Chen X, Schnell DJ. Chen K, et al. Biochem Soc Trans. 2000;28(4):485-91. Biochem Soc Trans. 2000. PMID: 10961945 Review. - Chloroplast precursor protein translocon.
May T, Soll J. May T, et al. FEBS Lett. 1999 Jun 4;452(1-2):52-6. doi: 10.1016/s0014-5793(99)00527-x. FEBS Lett. 1999. PMID: 10376677 Review.
Cited by
- Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif.
Robert V, Volokhina EB, Senf F, Bos MP, Van Gelder P, Tommassen J. Robert V, et al. PLoS Biol. 2006 Nov;4(11):e377. doi: 10.1371/journal.pbio.0040377. PLoS Biol. 2006. PMID: 17090219 Free PMC article. - A molecular-genetic study of the Arabidopsis Toc75 gene family.
Baldwin A, Wardle A, Patel R, Dudley P, Park SK, Twell D, Inoue K, Jarvis P. Baldwin A, et al. Plant Physiol. 2005 Jun;138(2):715-33. doi: 10.1104/pp.105.063289. Epub 2005 May 20. Plant Physiol. 2005. PMID: 15908591 Free PMC article. - Origins, function, and regulation of the TOC-TIC general protein import machinery of plastids.
Richardson LGL, Schnell DJ. Richardson LGL, et al. J Exp Bot. 2020 Feb 19;71(4):1226-1238. doi: 10.1093/jxb/erz517. J Exp Bot. 2020. PMID: 31730153 Free PMC article. - Chloroplast Outer Membrane β-Barrel Proteins Use Components of the General Import Apparatus.
Day PM, Inoue K, Theg SM. Day PM, et al. Plant Cell. 2019 Aug;31(8):1845-1855. doi: 10.1105/tpc.19.00001. Epub 2019 Jun 19. Plant Cell. 2019. PMID: 31217220 Free PMC article. - Bacterial origin of a mitochondrial outer membrane protein translocase: new perspectives from comparative single channel electrophysiology.
Harsman A, Niemann M, Pusnik M, Schmidt O, Burmann BM, Hiller S, Meisinger C, Schneider A, Wagner R. Harsman A, et al. J Biol Chem. 2012 Sep 7;287(37):31437-45. doi: 10.1074/jbc.M112.392118. Epub 2012 Jul 9. J Biol Chem. 2012. PMID: 22778261 Free PMC article.
References
- Margulis L. Origin of Eukaryotic Cells. New Haven, CT: Yale Univ. Press; 1970.
- Martin W, Müller M. Nature (London) 1998;392:37–41. - PubMed
- Cavalier-Smith T. Ann NY Acad Sci. 1987;503:55–71. - PubMed
- Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, Kowallik K V. Nature (London) 1998;393:162–165. - PubMed
- Heins L, Collinson I, Soll J. Trends Plant Sci. 1998;3:56–61.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources