Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse - PubMed (original) (raw)
Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse
J E Shea et al. Infect Immun. 1999 Jan.
Abstract
We have investigated the in vivo growth kinetics of a Salmonella typhimurium strain (P11D10) carrying a mutation in ssaJ, a Salmonella pathogenicity island 2 (SPI2) gene encoding a component of a type III secretion system required for systemic growth in mice. Similar numbers of mutant and wild-type cells were recovered from the spleens and livers of BALB/c mice up to 8 h after inoculation by the intraperitoneal route. Thereafter, the numbers of wild-type cells continued to increase logarithmically in these organs, whereas those of P11D10 remained relatively static for several days before being cleared. Gentamicin protection experiments on spleen cell suspensions recovered from infected mice showed that viable intracellular wild-type bacteria accumulated over time but that intracellular P11D10 cells did not. Infection experiments were also performed with wild-type and P11D10 cells carrying the temperature-sensitive plasmid pHSG422 to distinguish between bacterial growth rates and killing in vivo. At 16 h postinoculation there were 10-fold more wild-type cells than mutant cells in the spleens of infected mice, but the numbers of cells of both strains carrying the nonreplicating plasmid were very similar, showing that there was little difference in the degree of killing sustained by the two strains and that the SPI2 secretion system must be required for bacterial replication, rather than survival, in vivo. The SPI2 mutant phenotype in mice is similar to that of strains carrying mutations in the Salmonella virulence plasmid spv genes. To determine if these two sets of genes interact together, a double mutant strain carrying SPI2 and spv mutations was constructed and compared with strains carrying single mutations in terms of virulence attenuation. These experiments failed to provide any evidence showing that the SPI2 and spv gene products interact together as part of the same virulence mechanism.
Figures
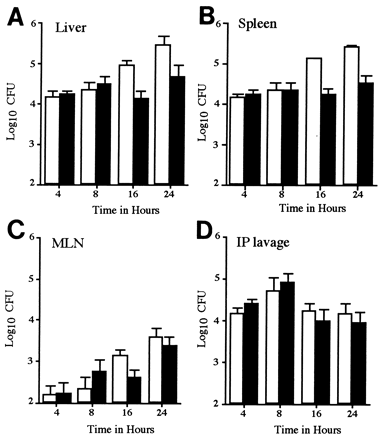
FIG. 1
In vivo growth kinetics of a SPI2 mutant strain in a mixed infection. Mice were inoculated i.p. with 105 CFU of a mixed inoculum of 12023 and P11D10 strains. The numbers of 12023 (open bars) and P11D10 (filled bars) bacterial CFU in the liver (A), spleen (B), MLN (C), and peritoneal lavage (D) were determined at various time points postinoculation. Data shown are the means ± standard deviation (SD) for groups of 5 mice and are normalized to a 50:50 ratio in the inoculum.
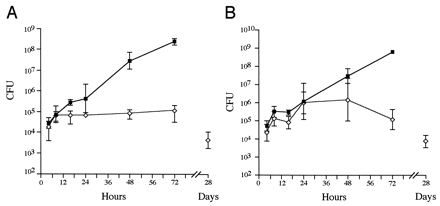
FIG. 2
In vivo growth kinetics in single-strain infections. The bacterial loads in the spleen (A) and the liver (B) were determined over time for single infections with 12023 (squares) and P11D10 (diamonds). Data are expressed as the means ± 1 SD for groups of 5 mice.
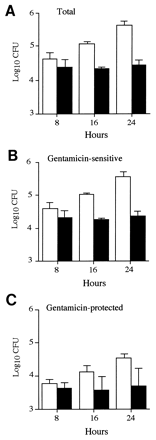
FIG. 3
Gentamicin protection assays of spleen cell suspensions after mixed infections. The total numbers of P11D10 (solid bars) and wild-type (open bars) bacteria within the spleen (A), as well as the number of these that were gentamicin-sensitive (B) or protected (C), were determined over time in mixed infections. Data are expressed as the mean ± 1 SD for groups of 5 mice.
Similar articles
- Salmonella typhimurium strains carrying independent mutations display similar virulence phenotypes yet are controlled by distinct host defense mechanisms.
Raupach B, Kurth N, Pfeffer K, Kaufmann SH. Raupach B, et al. J Immunol. 2003 Jun 15;170(12):6133-40. doi: 10.4049/jimmunol.170.12.6133. J Immunol. 2003. PMID: 12794143 - Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages.
Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang FC, Holden DW. Hensel M, et al. Mol Microbiol. 1998 Oct;30(1):163-74. doi: 10.1046/j.1365-2958.1998.01047.x. Mol Microbiol. 1998. PMID: 9786193 - Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo.
Beuzón CR, Holden DW. Beuzón CR, et al. Microbes Infect. 2001 Nov-Dec;3(14-15):1345-52. doi: 10.1016/s1286-4579(01)01496-4. Microbes Infect. 2001. PMID: 11755424 Review. - Identification and analysis of bacterial virulence genes in vivo.
Unsworth KE, Holden DW. Unsworth KE, et al. Philos Trans R Soc Lond B Biol Sci. 2000 May 29;355(1397):613-22. doi: 10.1098/rstb.2000.0602. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10874734 Free PMC article. Review.
Cited by
- Selective culling of high avidity antigen-specific CD4+ T cells after virulent Salmonella infection.
Ertelt JM, Johanns TM, Mysz MA, Nanton MR, Rowe JH, Aguilera MN, Way SS. Ertelt JM, et al. Immunology. 2011 Dec;134(4):487-97. doi: 10.1111/j.1365-2567.2011.03510.x. Immunology. 2011. PMID: 22044420 Free PMC article. - Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice.
Auerbuch V, Lenz LL, Portnoy DA. Auerbuch V, et al. Infect Immun. 2001 Sep;69(9):5953-7. doi: 10.1128/IAI.69.9.5953-5957.2001. Infect Immun. 2001. PMID: 11500481 Free PMC article. - Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis.
Coombes BK, Coburn BA, Potter AA, Gomis S, Mirakhur K, Li Y, Finlay BB. Coombes BK, et al. Infect Immun. 2005 Nov;73(11):7161-9. doi: 10.1128/IAI.73.11.7161-7169.2005. Infect Immun. 2005. PMID: 16239510 Free PMC article. - Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates.
Chakravortty D, Hansen-Wester I, Hensel M. Chakravortty D, et al. J Exp Med. 2002 May 6;195(9):1155-66. doi: 10.1084/jem.20011547. J Exp Med. 2002. PMID: 11994420 Free PMC article. - Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes.
Srinivasan A, Nanton M, Griffin A, McSorley SJ. Srinivasan A, et al. J Immunol. 2009 Jun 15;182(12):7838-45. doi: 10.4049/jimmunol.0900382. J Immunol. 2009. PMID: 19494308 Free PMC article.
References
- Benjamin W H, Jr, Hall P, Roberts S J, Briles D E. The primary effect of the ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J Immunol. 1990;144:3143–3151. - PubMed
- Carrol M E W, Jackett P S, Aber V R, Lowrie D B. Phagolysosome formation, cyclic adenosine 3′:5′-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979;110:421–429. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous