The polysaccharide portion of lipopolysaccharide regulates antigen-specific T-cell activation via effects on macrophage-mediated antigen processing - PubMed (original) (raw)
The polysaccharide portion of lipopolysaccharide regulates antigen-specific T-cell activation via effects on macrophage-mediated antigen processing
N M Zirk et al. Infect Immun. 1999 Jan.
Abstract
The lipopolysaccharide (LPS) structure of Salmonella typhimurium has been correlated with the virulence of wild-type strain LT2. Mutants of LT2 with truncated polysaccharide portions of LPS are less virulent than strains with a complete LPS structure. Polyclonal T cells and monoclonal T-cell hybridomas were more reactive to heat-killed rough mutants than to heat-killed smooth strains, as measured by interleukin-2 (IL-2) production. Using a large panel of strains with truncated LPS molecules, we found that T-cell reactivity decreased with certain lengths of polysaccharide. The decreased response was not due to differential phagocytic uptake, IL-12 production, or major histocompatibility complex class II surface expression by macrophages. Also, LT2 did not mediate any global suppression since addition of LT2 did not diminish the response of T cells specific for antigens unrelated to Salmonella. In an experiment in which processing times were varied, we found that antigens from rough strains were processed and presented more quickly than those associated with smooth strains. At longer processing times, epitopes from LT2 were presented well. We hypothesize that the slower antigen processing and presentation of wild-type Salmonella may be caused by masking of surface antigens by the longer polysaccharide portion of smooth LPS. This blocking of effective antigen presentation may contribute to the virulence of Salmonella.
Figures
FIG. 1
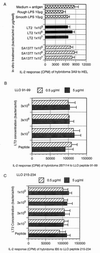
T cells from the PEC population respond to rough strains of heat-killed S. typhimurium better than to smooth strains in a dose-dependent manner. PEC from mice immunized with 108 live SL1004 organisms per mouse were combined with 107 heat-killed bacteria per ml in 96-well microtiter plates at a concentration of 3 × 105/well for 24 h. The levels of IL-2 in the resulting supernatants were measured by using the IL-2-dependent cell line HT-2 as an indicator. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells.
FIG. 2
T cells in the PEC population from S. typhimurium SL1004-immunized mice respond to HKST SL1004 (107 organisms/ml), a rough LPS mutant, as the dose increases. The T cells are minimally responsive to wild-type heat-killed LT2. PEC (3 × 105/ml) were combined with 107 heat-killed bacteria per ml in 96-well microtiter plates for 24 h. The level of IL-2 in the resulting supernatants were measured by using the IL-2-dependent cell line HT-2 as an indicator. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells.
FIG. 3
A monoclonal T-cell hybridoma (18-15.18) responds to an antigen(s) from rough strains in a dose-dependent manner. Hybridoma 18-15.18 produces more IL-2 in the presence of LPS mutant strains than in the wild-type LT2 strain. The response increases as LPS length decreases. ConA-elicited macrophages (105/well) were used as APC and combined in a 96-well format with 105 T-cell hybridoma 18-15.18 cells and 107 HKST organisms per well for 24 h. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells.
FIG. 4
T-cell hybridoma 18-15.18 responds to live rough LPS mutant bacteria better than to live smooth wild-type bacteria in a dose-dependent manner. ConA-elicited macrophages (105/well) were incubated with live, PBS-washed S. typhimurium bacteria in various concentrations for 2 h. The extracellular bacteria were then removed, and the macrophages were fixed with glutaraldehyde. The T-cell hybridoma was added (105 cells/well) and incubated for 24 h. The IL-2 production in the supernatants was measured. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells.
FIG. 5
(A) The presence of purified LPS or HKST does not suppress the ability of T-cell hybridoma 3A9 to respond to hen egg lysozyme. (B) Wild-type LT2 does not abrogate the MHC class I-restricted response of T-cell hybridoma 2B7114 to listeriolysin O (LLO) peptide 91–99. (C) LT2 does not diminish the MHC class II-restricted response of T-cell hybridoma IB5 to listeriolysin O (LLO) peptide 215–234. Purified LPS, heat-killed LT2, heat-killed SA1377, and peptide were added together in various concentrations to hybridoma (105 cells/well) and ConA-elicited macrophages (105/well) in a 96-well format and incubated 24 h. The supernatants were evaluated for IL-2 by using the HT-2 cell line as an indicator. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells. Because hybridomas are specific, cross reactivity did not interfere with the response as shown.
FIG. 6
Uptake of the wild-type strain and uptake of LPS mutant strains of S. typhimurium by macrophages are similar. (A) Bacteria were labeled overnight with 20 μCi of [3H]thymidine per ml, washed three times with PBS, and heat killed (80°C for 1 h). The bacteria were added to ConA-elicited macrophages in a 96-well format at a ratio of 10:1. Macrophages were lysed at intervals with 0.05% Triton X-100, and the lysate was dried onto a Spot Plate (Packard). Radioactivity was determined in a Matrix 96 direct beta counter. The number of bacteria were calculated from a standard curve (relating counts per minute and the number of bacterial for each strain) generated from dilutions of heat-killed bacteria dried onto the Spot Plates. (B) Uptake of live S. typhimurium by ConA-elicited macrophages. Macrophages were cultured with live bacteria at a ratio of 1:100 for 1 to 4 h and then lysed with Triton X-100 at each time indicated. Dilutions of input and recovered bacteria were plated on BHI agar for counting.
FIG. 7
Epitopes for T-cell activation are present in the smooth strain and are revealed over longer processing times. The response of T-cell hybridoma 18-15.18 to heat-killed S. typhimurium LT2 in vitro increases after 24 h of processing. Heat-killed bacteria (107/ml) were incubated with ConA-elicited macrophages (105/well) for various intervals. At each time indicated, macrophages were washed and fixed with 0.05% glutaraldehyde. T cells (hybridoma 18-15.18 [105 cells/well]) were added for 24 h. IL-2 production in supernatants was measured. The IL-2 response, recorded in counts per minute (CPM), is expressed as the means ± SD (error bars) for triplicate wells.
References
- Allen P M, Unanue E R. Processing and presentation of hen egg-white lysozyme by macrophages. Immunobiology. 1984;168:182–188. - PubMed
- Al-Ramadi B K, Brodkin M A, Mosser D M, Eisenstein T K. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–2746. - PubMed
- Arai T, Matsui K. A purified protein from Salmonella typhimurium inhibits high-affinity interleukin-2 receptor expression on CTLL-2 cells. FEMS Immunol Med Microbiol. 1997;17:155–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources