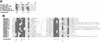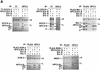The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families - PubMed (original) (raw)
The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families
T Kamura et al. Genes Dev. 1998.
Abstract
The Elongin BC complex was identified initially as a positive regulator of RNA polymerase II (Pol II) elongation factor Elongin A and subsequently as a component of the multiprotein von Hippel-Lindau (VHL) tumor suppressor complex, in which it participates in both tumor suppression and negative regulation of hypoxia-inducible genes. Elongin B is a ubiquitin-like protein, and Elongin C is a Skp1-like protein that binds to a BC-box motif that is present in both Elongin A and VHL and is distinct from the conserved F-box motif recognized by Skp1. In this report, we demonstrate that the Elongin BC complex also binds to a functional BC box present in the SOCS box, a sequence motif identified recently in the suppressor of cytokine signaling-1 (SOCS-1) protein, as well as in a collection of additional proteins belonging to the SOCS, ras, WD-40 repeat, SPRY domain, and ankyrin repeat families. In addition, we present evidence (1) that the Elongin BC complex is a component of a multiprotein SOCS-1 complex that attenuates Jak/STAT signaling by binding to Jak2 and inhibiting Jak2 kinase, and (2) that by interacting with the SOCS box, the Elongin BC complex can increase expression of the SOCS-1 protein by inhibiting its degradation. These results suggest that Elongin BC is a multifunctional regulatory complex capable of controlling multiple pathways in the cell through interaction with a short degenerate sequence motif found in many different proteins.
Figures
Figure 1
Partial purification of Elongin BC-containing complexes from rat liver. Homogenate was prepared from the livers of 10 male Sprague–Dawley rats (200–250 grams) and subjected to subcellular fractionation, ammonium sulfate precipitation, and phosphocellulose chromatography as described (Conaway et al. 1996). Ten microliters of each fraction was subjected to SDS-PAGE along with the indicated amounts of recombinant histidine-tagged Elongin A (6His–EloA), histidine- and FLAG-tagged VHL (6His–FLAG–VHL), or histidine- and HPC4-tagged Elongin B (6His–HPC4–EloB) and then immunoblotted with anti-Elongin A, anti-VHL, and anti-Elongin B antibodies. The 0.1, 0.3, 0.6, and 1
m
KCl eluates from phosphocellulose are indicated as 0.1, 0.3, 0.6, and 1.0, respectively. The volumes of the fractions shown in lanes 5–8 were 50, 25, 25, and 20 ml; fractions in lanes 9–12 were 200, 150, 120, and 120 ml; fractions in lanes 17–20 were 15, 10, 10, and 10 ml; and fractions in lanes 21–24 were 40, 20, 15, and 15 ml. (AmSO4) Ammonium sulfate; (EloA) Elongin A; (EloB) Elongin B; (P-11) phosphocellulose.
Figure 2
The SOCS box includes a consensus Elongin BC binding site. (A) Sequence alignment of the Elongin BC binding site regions of VHL and Elongin A proteins from mammalian cells, C. elegans, and S. cerevisiae. (B) The SOCS-box regions of several SOCS-box proteins (Hilton et al. 1998) are aligned beneath Elongin BC binding sites from VHL and Elongin A proteins. (C) Diagrammatic representation of Elongin A, VHL, and the SOCS-box proteins used in this study.
Figure 2
The SOCS box includes a consensus Elongin BC binding site. (A) Sequence alignment of the Elongin BC binding site regions of VHL and Elongin A proteins from mammalian cells, C. elegans, and S. cerevisiae. (B) The SOCS-box regions of several SOCS-box proteins (Hilton et al. 1998) are aligned beneath Elongin BC binding sites from VHL and Elongin A proteins. (C) Diagrammatic representation of Elongin A, VHL, and the SOCS-box proteins used in this study.
Figure 3
Binding of the Elongin BC complex to SOCS-box proteins. (A) Recombinant proteins were expressed in E. coli and purified from guanidine-solubilized inclusion bodies by Ni2+–agarose affinity chromatography. Untagged or epitope-tagged SOCS-1, SOCS-3, Rar-1, WSB-1, ASB-1, Elongin B, and Elongin C were mixed together in the combinations indicated, renatured by dilution and dialysis, and immunoprecipitated with the antibodies indicated. Following SDS-PAGE, immunoprecipitated proteins were visualized by Coomassie Blue staining. (B) T7-tagged SOCS-1 and SOCS-3, Flag-tagged WSB-1, ASB-2, and RAR-1 were in vitro transcribed and translated together with HPC-4 tagged Elongin B and HSV-tagged Elongin C by use of the Promega TNT/T7 rabbit reticulocyte lysate kit with [35S]methionine according to the manufacturer’s instructions. In vitro-translated proteins were immunoprecipitated with the antibodies indicated, subjected to SDS-PAGE, and visualized with a PhosphorImager (Molecular Dynamics). (H,L) Immunoglobulin heavy and light chains, respectively. (IP) Immunoprecipitate; (WB) Western blot; (F) Flag epitope; (B) Elongin B; (C) Elongin C.
Figure 3
Binding of the Elongin BC complex to SOCS-box proteins. (A) Recombinant proteins were expressed in E. coli and purified from guanidine-solubilized inclusion bodies by Ni2+–agarose affinity chromatography. Untagged or epitope-tagged SOCS-1, SOCS-3, Rar-1, WSB-1, ASB-1, Elongin B, and Elongin C were mixed together in the combinations indicated, renatured by dilution and dialysis, and immunoprecipitated with the antibodies indicated. Following SDS-PAGE, immunoprecipitated proteins were visualized by Coomassie Blue staining. (B) T7-tagged SOCS-1 and SOCS-3, Flag-tagged WSB-1, ASB-2, and RAR-1 were in vitro transcribed and translated together with HPC-4 tagged Elongin B and HSV-tagged Elongin C by use of the Promega TNT/T7 rabbit reticulocyte lysate kit with [35S]methionine according to the manufacturer’s instructions. In vitro-translated proteins were immunoprecipitated with the antibodies indicated, subjected to SDS-PAGE, and visualized with a PhosphorImager (Molecular Dynamics). (H,L) Immunoglobulin heavy and light chains, respectively. (IP) Immunoprecipitate; (WB) Western blot; (F) Flag epitope; (B) Elongin B; (C) Elongin C.
Figure 4
Binding of the Elongin BC complex to SOCS-1 is disrupted by mutation of the SOCS-box Elongin BC binding site motif. (Lanes 1–3) Twenty-five micrograms of total cell lysate from Sf21 cells infected with the baculovirus vectors encoding HPC4-tagged Elongin B, HSV-tagged Elongin C, and the indicated wild-type or mutant T7-tagged SOCS-1 proteins was immunoblotted with anti-T7, anti-HPC4, or anti-HSV antibodies. (Lanes 4–12) Three hundred micrograms of total cell lysate protein from baculovirus-infected Sf21 cells was immunopreciptiated with antibodies specific for T7 epitope (IP: T7-SOCS-1), HPC4 epitope (IP: HPC4-EloB), or HSV epitope (IP: HSV-EloC) and then immunoblotted with anti-T7, anti-HPC4, or anti-HSV antibodies. (H,L) Immunoglobulin heavy and light chains, respectively.
Figure 5
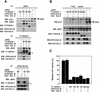
Elongins B and C are components of the SOCS-1 complex but are not required for inhibition of Jak activity. 293T cells were transiently transfected with the indicated combinations of Jak2, wild-type or mutant T7–SOCS-1, HPC4–Elongin B, and HSV–Elongin C expression vectors. The amount of the T7–SOCS-1 expression vectors used in each transfection was adjusted so that comparable levels of wild-type SOCS-1 and SOCS-1 mutants would be expressed. (A) Three hundred micrograms of total cell lysate protein from transfected cells was immunopreciptiated with antibodies specific for Jak2 (IP: Jak2), T7 epitope (IP: T7-SOCS-1), or HPC4 epitope (IP: HPC4-EloB) and then immunoblotted with anti-pTyr, anti-Jak2, anti-T7, anti-HPC4, or anti-HSV antibodies. (L) Immunoglobulin light chain. (B) Twenty-five micrograms of the indicated total cell lysates was immunoblotted with the antibodies indicated. (C) Hep3B cells were transiently transfected in 35-mm dishes with 1 μg each of the IL6-inducible STAT-dependent CAT reporter construct TIK (gift of W. Liao, M.D. Anderson Cancer Center, Houston, TX) and the β-galactosidase control reporter pCMVβ (Clontech), with or without 1 μg of expression vectors encoding T7-tagged SOCS-1, HPC4-tagged Elongin B, and HSV-tagged Elongin C. After 40 hr, cells were stimulated with 100 ng/ml IL-6 for 3 hr, and cell extracts were assayed for CAT and β-galactosidase activities with the CAT and β-galactosidase assay systems from Promega. CAT activity, normalized to β-galactosidase activity, is expressed as the percent of that observed in lysates from cells not transfected with SOCS-1, Elongin B, and Elongin C expression vectors.
Figure 6
Interaction of SOCS-Box proteins with Elongin BC. (A) Hep3B cells stably transformed with T7-SOCS-1 (lane 1) or empty control vector (lane 2) were incubated for 9 hr with 100 μ
m
ZnCl2 in DMEM, 10% fetal calf serum prior to harvesting and cell lysis. Two-hundred micrograms of total cell lysate protein was immunoprecipitated with anti-T7 antibodies and then immunoblotted with anti-T7, anti-Elongin B, or anti-Elongin C antibodies. (B) NIH-3T3 cells were stimulated with 1000 units of γ-interferon for 5 hr before harvesting and cell lysis (lanes 1–4). M1 cells were stimulated with 50 ng/ml IL-6 for 5 hr before harvesting and cell lysis (lanes 5–7). Approximately 5 mg of total cell lysate protein was immunoprecipitated with the indicated antibodies and then immunoblotted with anti-SOCS-1 (N18) (lanes 1–2), anti-SOCS-3 (S19) (lanes 3–4), and anti-Elongin B and anti-Elongin C antibodies as indicated.
Figure 7
Interaction of Elongin BC with SOCS-1 increases its stability. 293T cells were transiently transfected in 35-mm dishes with 1 μg of the HPC4-Elongin B expression vector, 1 μg of the HSV-Elongin C expression vector, and 1 μg of the wild-type T7-SOCS-1 expression vector or 3 μg of the T7-SOCS-1 M1 expression vector as indicated. (A) Cycloheximide block. Twenty-four hours after transfection, 100 μg/ml cycloheximide was added to culture dishes. Cells were harvested and lysed after an additional incubation for the indicated times. Twenty-five micrograms of total cell lysate protein was immunoblotted with the indicated antibodies. SOCS-1 protein was quantitated by densitometry of Western blots using NIH Image 1.60/ppc. (B) [35S]methionine pulse chase. Cultures were washed twice with PBS and incubated with methionine-free medium containing 10% dialyzed fetal calf serum for 1 hr prior to addition of [35S]methionine (0.5 mCi/ml of medium; EXPRE35S35S protein labeling mix, NEN), followed by an additional 2 hr incubation. The cells were then washed twice with PBS and cultured in DMEM with 10% fetal calf serum. Cells were harvested and lysed after an additional incubation for the indicated times. Approximately 200 μg of total cell lysate protein was immunoprecipitated with T7 antibody and analyzed by SDS-PAGE. Radioactive proteins were detected and quantitated by PhosphorImager analysis. The data plotted represents the mean of three independent experiments. Error bars indicate the standard error of the mean.
Figure 7
Interaction of Elongin BC with SOCS-1 increases its stability. 293T cells were transiently transfected in 35-mm dishes with 1 μg of the HPC4-Elongin B expression vector, 1 μg of the HSV-Elongin C expression vector, and 1 μg of the wild-type T7-SOCS-1 expression vector or 3 μg of the T7-SOCS-1 M1 expression vector as indicated. (A) Cycloheximide block. Twenty-four hours after transfection, 100 μg/ml cycloheximide was added to culture dishes. Cells were harvested and lysed after an additional incubation for the indicated times. Twenty-five micrograms of total cell lysate protein was immunoblotted with the indicated antibodies. SOCS-1 protein was quantitated by densitometry of Western blots using NIH Image 1.60/ppc. (B) [35S]methionine pulse chase. Cultures were washed twice with PBS and incubated with methionine-free medium containing 10% dialyzed fetal calf serum for 1 hr prior to addition of [35S]methionine (0.5 mCi/ml of medium; EXPRE35S35S protein labeling mix, NEN), followed by an additional 2 hr incubation. The cells were then washed twice with PBS and cultured in DMEM with 10% fetal calf serum. Cells were harvested and lysed after an additional incubation for the indicated times. Approximately 200 μg of total cell lysate protein was immunoprecipitated with T7 antibody and analyzed by SDS-PAGE. Radioactive proteins were detected and quantitated by PhosphorImager analysis. The data plotted represents the mean of three independent experiments. Error bars indicate the standard error of the mean.
Figure 8
Multiple functions for the Elongin BC complex.
Similar articles
- The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation.
Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, Kile BT, Kent SB, Alexander WS, Metcalf D, Hilton DJ, Nicola NA, Baca M. Zhang JG, et al. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2071-6. doi: 10.1073/pnas.96.5.2071. Proc Natl Acad Sci U S A. 1999. PMID: 10051596 Free PMC article. - A three-dimensional model of Suppressor Of Cytokine Signalling 1 (SOCS-1).
Giordanetto F, Kroemer RT. Giordanetto F, et al. Protein Eng. 2003 Feb;16(2):115-24. doi: 10.1093/proeng/gzg015. Protein Eng. 2003. PMID: 12676980 - Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase.
Kamura T, Burian D, Yan Q, Schmidt SL, Lane WS, Querido E, Branton PE, Shilatifard A, Conaway RC, Conaway JW. Kamura T, et al. J Biol Chem. 2001 Aug 10;276(32):29748-53. doi: 10.1074/jbc.M103093200. Epub 2001 May 30. J Biol Chem. 2001. PMID: 11384984 - Role of SOCS and VHL Proteins in Neuronal Differentiation and Development.
Kanno H, Matsumoto S, Yoshizumi T, Nakahara K, Kubo A, Murata H, Shuin T, U HS. Kanno H, et al. Int J Mol Sci. 2023 Feb 15;24(4):3880. doi: 10.3390/ijms24043880. Int J Mol Sci. 2023. PMID: 36835292 Free PMC article. Review. - The suppressors of cytokine signalling (SOCS).
Kile BT, Alexander WS. Kile BT, et al. Cell Mol Life Sci. 2001 Oct;58(11):1627-35. doi: 10.1007/PL00000801. Cell Mol Life Sci. 2001. PMID: 11706989 Free PMC article. Review.
Cited by
- The structure and regulation of Cullin 2 based E3 ubiquitin ligases and their biological functions.
Cai W, Yang H. Cai W, et al. Cell Div. 2016 May 23;11:7. doi: 10.1186/s13008-016-0020-7. eCollection 2016. Cell Div. 2016. PMID: 27222660 Free PMC article. Review. - The role of the cullin-5 e3 ubiquitin ligase in the regulation of insulin receptor substrate-1.
Hu CZ, Sethi JK, Hagen T. Hu CZ, et al. Biochem Res Int. 2012;2012:282648. doi: 10.1155/2012/282648. Epub 2012 Dec 9. Biochem Res Int. 2012. PMID: 23304509 Free PMC article. - SOCS1 is a critical checkpoint in immune homeostasis, inflammation and tumor immunity.
Bidgood GM, Keating N, Doggett K, Nicholson SE. Bidgood GM, et al. Front Immunol. 2024 Jun 14;15:1419951. doi: 10.3389/fimmu.2024.1419951. eCollection 2024. Front Immunol. 2024. PMID: 38947335 Free PMC article. Review. - SOCS, SPRED, and NR4a: Negative regulators of cytokine signaling and transcription in immune tolerance.
Yoshimura A, Aki D, Ito M. Yoshimura A, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(6):277-291. doi: 10.2183/pjab.97.016. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 34121041 Free PMC article. - Suppressor of Cytokine Signaling 3 Is an Inducible Host Factor That Regulates Virus Egress during Ebola Virus Infection.
Okumura A, Rasmussen AL, Halfmann P, Feldmann F, Yoshimura A, Feldmann H, Kawaoka Y, Harty RN, Katze MG. Okumura A, et al. J Virol. 2015 Oct;89(20):10399-406. doi: 10.1128/JVI.01736-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246577 Free PMC article.
References
- Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285–1287. - PubMed
- Aso T, Conrad MN. Molecular cloning of DNAs encoding the regulatory subunits of elongin from Saccharomyces cerevisiae and Drosophila melanogaster. Biochem Biophys Res Commun. 1997;241:334–340. - PubMed
- Aso T, Lane WS, Conaway JW, Conaway RC. Elongin (SIII): A multisubunit regulator of elongation by RNA polymerase II. Science. 1995;269:1439–1443. - PubMed
- Auerenhammer CJ, Chesnokova V, Bousqet C, Melmed S. Pituitary corticotroph SOCS-3: A novel intracellular regulation of leukemia-inhibitory factor-mediated proopiomelanocortin gene expression and adrenocorticotropin secretion. Mol Endocrinol. 1998;12:954–961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous