Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures - PubMed (original) (raw)
Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures
H Eng et al. J Neurosci. 1999.
Abstract
The proteins needed for growth and maintenance of the axon are generally believed to be synthesized in the cell bodies and delivered to the axons by anterograde transport. However, recent reports suggest that some proteins can also be synthesized within axons. We used [35S]methionine metabolic labeling to investigate axonal protein synthesis in compartmented cultures of sympathetic neurons from newborn rats. Incubation of distal axons for 4 hr with [35S]methionine resulted in a highly specific pattern of labeled axonal proteins on SDS-PAGE, with 4 prominent bands in the 43-55 kDa range. The labeled proteins in axons were not synthesized in the cell bodies, because they were also produced by axons after the cell bodies had been removed. Two of the proteins were identified by immunoprecipitation as actin and beta-tubulin. Axons synthesized <1% of the actin and tubulin synthesized in the cell bodies and transported into the axons, and 75-85% inhibition of axonal protein synthesis by cycloheximide and puromycin failed to inhibit axonal elongation. Nonetheless, the specific production by axons of the major proteins of the axonal cytoskeleton suggests that axonal protein synthesis arises from specific mechanisms and likely has biological significance. One hypothetical scenario involves neurons with long axons in vivo in which losses from turnover during axonal transport may limit the availability of cell body synthesized proteins to the distal axons. In this case, a significant fraction of axonal proteins might be supplied by axonal synthesis, which could, therefore, play important roles in axonal maintenance, regeneration, and sprouting.
Figures
Fig. 1.
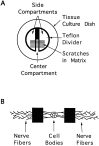
Schematic diagram of a compartmented culture.A, A Teflon divider provides a barrier that divides the culture dish into one central compartment and two side compartments.B, An enlargement of a single track shows the cell bodies and proximal axons restricted to the center compartment, whereas the distal axons cross under the barrier and extend into the side compartments.
Fig. 2.
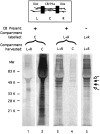
Comparison of the profiles of proteins labeled by incubation with [35S]met in left and right compartments or center compartments. Lanes 1,2, The center compartments of cultures were incubated for 4 hr with 250 μCi/ml [35S]met before harvesting the left and right (L+R) and center compartments (C). Extracts were analyzed by SDS-PAGE as described in Materials and Methods. Protein from three cultures was loaded on each lane. Lanes 3,4, To assess the possibility of protein synthesis in distal axons, left and right compartments of cultures were incubated with [35S]met for 4 hr, and the cultures were analyzed. Protein from 10 cultures was loaded on each lane. Lane 5, To determine whether the labeled proteins detected in axons incubated with [35S]met were transported there after synthesis in the cell bodies, the cell bodies were removed from cultures immediately before labeling the distal axons for 4 hr with [35S]met. Protein from 10 cultures was loaded on this lane.
Fig. 3.
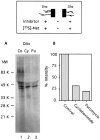
Inhibition of axonal protein synthesis. Cultures were preincubated 2 hr with culture medium in the distal axon compartments containing either cycloheximide (100 μg/ml;Cy), puromycin (120 μg/ml; Pu), or no drug as control (Co). Then, the distal compartment medium was replaced with [35S]met labeling medium containing the inhibitor or no inhibitor as appropriate, and the cultures were incubated for 4 hr. Proteins were harvested and analyzed by SDS-PAGE as described in Materials and Methods. Protein from six cultures was loaded on each lane. A, Lanes from the distal axons (DAx). B, Density of the individual lanes from A quantitated by phosphorimaging and expressed as a percentage of the control lane. C, Lanes from the cell bodies and proximal axons (CB/PAx).
Fig. 4.
Inhibition of axonal protein synthesis by direct application of cycloheximide and puromycin in isolated axons. The center compartments were washed with distilled water to remove the cell bodies before labeling. Control axons (Co) were not treated with drugs. Application of cycloheximide (Cy) and puromycin (Pu), labeling of axons, and analysis were as in Figure 3. Protein from six cultures was loaded on each lane.
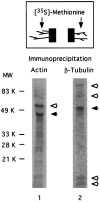
Fig. 5.
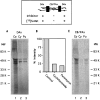
Immunoprecipitation of actin and β-tubulin synthesized in distal axons. After removal of the cell bodies, axons were incubated in [35S]met for 4 hr. Cell lysates from six cultures were immunoprecipitated with either anti-actin or anti-β-tubulin antibodies as described in Materials and Methods. Immunoprecipitated proteins were analyzed by SDS-PAGE and phosphorimaging. Anti-actin antibodies precipitated actin at 43 kDa (filled arrow) and coprecipitated one major band at 56 kDa (open arrow), as well as several other minor bands of varying molecular weight. Anti-β-tubulin antibodies precipitated β-tubulin (55 kDa, filled arrow), as well as four other bands (open arrows) of molecular weight 85, 75, 14, and 12 kDa.
Fig. 6.
Effect of protein synthesis inhibitors on axonal growth. Cycloheximide (100 μg/ml; A) or puromycin (120 μg/ml; B) was applied directly to axons in the right compartments of intact cultures, and medium without inhibitor was applied to axons in the left compartments as control. The growth of axons after introduction of the inhibitors was measured after 6 and 30 hr as described in Materials and Methods. The mean ± SE axonal extension for each time point is shown; n = 15–34 tracks.
Similar articles
- Delivery of newly synthesized tubulin to rapidly growing distal axons of sympathetic neurons in compartmented cultures.
Campenot B, Lund K, Senger DL. Campenot B, et al. J Cell Biol. 1996 Nov;135(3):701-9. doi: 10.1083/jcb.135.3.701. J Cell Biol. 1996. PMID: 8909544 Free PMC article. - Block of slow axonal transport and axonal growth by brefeldin A in compartmented cultures of rat sympathetic neurons.
Campenot RB, Soin J, Blacker M, Lund K, Eng H, MacInnis BL. Campenot RB, et al. Neuropharmacology. 2003 Jun;44(8):1107-17. doi: 10.1016/s0028-3908(03)00042-x. Neuropharmacology. 2003. PMID: 12763103 - A HaloTag® method for assessing the retrograde axonal transport of the p75 neurotrophin receptor and other proteins in compartmented cultures of rat sympathetic neurons.
Mok SA, Lund K, Lapointe P, Campenot RB. Mok SA, et al. J Neurosci Methods. 2013 Mar 30;214(1):91-104. doi: 10.1016/j.jneumeth.2013.01.006. Epub 2013 Jan 21. J Neurosci Methods. 2013. PMID: 23348044 - Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration.
Bisby MA, Tetzlaff W. Bisby MA, et al. Mol Neurobiol. 1992 Summer-Fall;6(2-3):107-23. doi: 10.1007/BF02780547. Mol Neurobiol. 1992. PMID: 1476674 Review. - Axonal transport of tubulin and actin.
Galbraith JA, Gallant PE. Galbraith JA, et al. J Neurocytol. 2000 Nov-Dec;29(11-12):889-911. doi: 10.1023/a:1010903710160. J Neurocytol. 2000. PMID: 11466477 Review.
Cited by
- The role of local protein synthesis and degradation in axon regeneration.
Gumy LF, Tan CL, Fawcett JW. Gumy LF, et al. Exp Neurol. 2010 May;223(1):28-37. doi: 10.1016/j.expneurol.2009.06.004. Epub 2009 Jun 9. Exp Neurol. 2010. PMID: 19520073 Free PMC article. Review. - Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity.
Donnelly CJ, Willis DE, Xu M, Tep C, Jiang C, Yoo S, Schanen NC, Kirn-Safran CB, van Minnen J, English A, Yoon SO, Bassell GJ, Twiss JL. Donnelly CJ, et al. EMBO J. 2011 Sep 30;30(22):4665-77. doi: 10.1038/emboj.2011.347. EMBO J. 2011. PMID: 21964071 Free PMC article. - Messenger RNAs localized to distal projections of human stem cell derived neurons.
Bigler RL, Kamande JW, Dumitru R, Niedringhaus M, Taylor AM. Bigler RL, et al. Sci Rep. 2017 Apr 4;7(1):611. doi: 10.1038/s41598-017-00676-w. Sci Rep. 2017. PMID: 28377585 Free PMC article. - Local gene regulation in radial glia: Lessons from across the nervous system.
D'Arcy BR, Silver DL. D'Arcy BR, et al. Traffic. 2020 Dec;21(12):737-748. doi: 10.1111/tra.12769. Epub 2020 Nov 1. Traffic. 2020. PMID: 33058331 Free PMC article. Review. - Protein synthetic machinery and mRNA in regenerating tips of spinal cord axons in lamprey.
Jin LQ, Pennise CR, Rodemer W, Jahn KS, Selzer ME. Jin LQ, et al. J Comp Neurol. 2016 Dec 1;524(17):3614-3640. doi: 10.1002/cne.24020. Epub 2016 May 19. J Comp Neurol. 2016. PMID: 27120118 Free PMC article.
References
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. I. Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93:1–12. - PubMed
- Campenot RB. Compartmented culture analysis of nerve growth. In: Stevenson B, Paul D, Gallin W, editors. Cell–cell interactions: a practical approach. IRL; Oxford: 1992. pp. 275–298.
- Campenot RB. Construction and use of compartmented cultures. In: Federoff S, Richardson A, editors. Protocols for neural cell culture, Ed 2. Humana; Totowa, NJ: 1997. pp. 107–116.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources