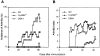Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis - PubMed (original) (raw)
Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis
T Yuasa et al. J Exp Med. 1999.
Abstract
Autoimmune diseases, like rheumatoid arthritis, result from a dysregulation of the immune response culminating in hyperactivation of effector cells leading to immune-mediated injury. To maintain an appropriate immune response and prevent the emergence of autoimmune disease, activation signals must be regulated by inhibitory pathways. Biochemical and genetic studies indicate that the type IIB low-affinity receptor for immunoglobulin (Ig)G (FcgammaRIIB) inhibits cellular activation triggered through antibody or immune complexes and may be an important component in preventing the emergence of autoimmunity. To investigate the role of FcgammaRIIB in the development of type II collagen (CII)-induced arthritis (CIA), a model for rheumatoid arthritis in humans, we have examined its contribution in determining the susceptibility to CIA in the nonpermissive H-2(b) haplotype. H-2(b) mice immunized with bovine CII do not develop appreciable disease. In contrast, immunization of the FcgammaRIIB-deficient, H-2(b) mice with bovine CII induced CIA at an incidence of 42.2%. The maximal arthritis index of the FcgammaRIIB-deficient mice developing CIA (6.9 +/- 3.6) was comparable to that of DBA/1 mice (8.6 +/- 1.9), an H-2(q) strain susceptible for CIA induction. IgG1, IgG2a, and IgG2b antibody responses against CII were elevated in the FcgammaRIIB-deficient animals, especially in those mice showing arthritis, but less pronounced than DBA/1 mice. Histological examinations of the arthritic paws from FcgammaRIIB-deficient mice revealed that cartilage was destroyed and bone was focally eroded in association with marked lymphocyte and monocyte/macrophage infiltration, very similar to the pathologic findings observed in DBA/1 mice. These results indicate that a nonpermissive H-2(b) haplotype can be rendered permissive to CIA induction through deletion of FcgammaRIIB, suggesting that FcgammaRIIB plays a critical role in suppressing the induction of CIA.
Figures
Figure 1
Development of CIA in mice by disruption of FcγRIIB expression. Incidence of arthritis (A) and severity of clinical signs (B) in FcγRIIB−/− mice (•, n = 16), wild-type H-2b mice of 129/B6 hybrid background (⋄, n = 13), and DBA/1 mice (□, n = 11) after immunization with CII in CFA as described in Materials and Methods. Results are expressed as a percentage of arthritic mice with the arthritic index 5 (A) and as the mean arthritic scores in each group on a given day during the course of CIA (B). Representative data from three separate experiments with similar results are shown.
Figure 2
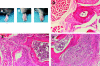
Clinical and histologic presentation of CIA in FcγRIIB−/− and DBA/1 mice. (A–C) The appearance of a normal forepaw from a CII-immunized wild-type mouse (A) contrasted with arthritic paws from an FcγRIIB−/− animal (B) and a positive control DBA/1 mouse (C). (D–F) Cross-sections of the forefoot from a normal wild-type mouse (D) compared with an arthritic joint from FcγRIIB−/− (E) and DBA/1 animals (F). Original magnifications: ×50 (D), ×80 (E), ×80 (F). D illustrates normal cartilage–bone without inflammation, whereas E and F show marked mononuclear cell infiltration with cartilage–bone destruction.
Figure 3
Concentration of anti-CII antibodies in sera from mice immunized with CII. The mean ± SD antibody levels of IgG1 (A), IgG2a (B), IgG2b (C), and IgM (D) subclasses, for all animals, and the mean ± SD of antibody levels (E–H) of arthritic DBA/1 (□) and FcγRIIB−/− (•) mice and of nonarthritic wild-type mice (⋄) are shown. **P < 0.01.
Figure 4
Proliferation and IFN-γ production of anticollagen lymph node cells in response to CII. (A) Lymph node cells (5 × 105/well) were stimulated in vitro with 5, 50, or 100 μg/ml heat-denatured CII (dCII) for 4 d. Proliferative response was determined by uptake of [3H]TdR pulsed for the final 18 h of culturing. (B) Each of the culture supernatants at the end of the experiment in A was collected and assessed for the IFN-γ content by ELISA. **P < 0.01 compared with wild-type mice.
Figure 5
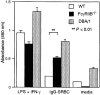
Secretion of IL-1α by peritoneal macrophages stimulated with IC. Thioglycollate-elicited peritoneal macrophages from FcγRIIB−/− (black bars) and wild-type (white bars) mice and DBA/1 mice (stippled bars) were stimulated with IgG1- opsonized SRBCs as described in Materials and Methods. The culture supernatant was analyzed for the IL-1α content by ELISA.
Similar articles
- Deregulation of peripheral B-cell development in enhanced severity of collagen-induced arthritis in FcgammaRIIB-deficient mice.
Nakamura A, Nukiwa T, Takai T. Nakamura A, et al. J Autoimmun. 2003 May;20(3):227-36. doi: 10.1016/s0896-8411(03)00034-9. J Autoimmun. 2003. PMID: 12753808 - IgG immune complex-binding in macrophages from arthritis-susceptible and arthritis-resistant mice following collagen type II immunization.
Thorvaldson L, Fuchs D, Magnusson S, Kleinau S. Thorvaldson L, et al. Scand J Immunol. 2006 May;63(5):347-54. doi: 10.1111/j.1365-3083.2006.01749.x. Scand J Immunol. 2006. PMID: 16640658 - The inhibitory co-receptor, PECAM-1 provides a protective effect in suppression of collagen-induced arthritis.
Wong MX, Hayball JD, Hogarth PM, Jackson DE. Wong MX, et al. J Clin Immunol. 2005 Jan;25(1):19-28. doi: 10.1007/s10875-005-0354-7. J Clin Immunol. 2005. PMID: 15742154 - A role of FcgammaRIIB in the development of collagen-induced arthritis.
Nakamura A, Takai T. Nakamura A, et al. Biomed Pharmacother. 2004 Jun;58(5):292-8. doi: 10.1016/j.biopha.2004.04.005. Biomed Pharmacother. 2004. PMID: 15194165 Review. - Immunopathogenesis of collagen arthritis.
Brand DD, Kang AH, Rosloniec EF. Brand DD, et al. Springer Semin Immunopathol. 2003 Aug;25(1):3-18. doi: 10.1007/s00281-003-0127-1. Springer Semin Immunopathol. 2003. PMID: 12904888 Review.
Cited by
- FcgammaRIIb inhibits allergic lung inflammation in a murine model of allergic asthma.
Dharajiya N, Vaidya SV, Murai H, Cardenas V, Kurosky A, Boldogh I, Sur SA. Dharajiya N, et al. PLoS One. 2010 Feb 22;5(2):e9337. doi: 10.1371/journal.pone.0009337. PLoS One. 2010. PMID: 20179765 Free PMC article. - A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs.
Anthony RM, Ravetch JV. Anthony RM, et al. J Clin Immunol. 2010 May;30 Suppl 1:S9-14. doi: 10.1007/s10875-010-9405-6. J Clin Immunol. 2010. PMID: 20480216 Review. - Fcγ Receptor IIB Controls Skin Inflammation in an Active Model of Epidermolysis Bullosa Acquisita.
Kovacs B, Tillmann J, Freund LC, Nimmerjahn F, Sadik CD, Bieber K, Ludwig RJ, Karsten CM, Köhl J. Kovacs B, et al. Front Immunol. 2020 Jan 14;10:3012. doi: 10.3389/fimmu.2019.03012. eCollection 2019. Front Immunol. 2020. PMID: 31993051 Free PMC article. - Sequence diversity of dengue virus type 2 in brain and thymus of infected interferon receptor ko mice: implications for dengue virulence.
Dhole P, Nakayama EE, Saito A, Limkittikul K, Phanthanawiboon S, Shioda T, Kurosu T. Dhole P, et al. Virol J. 2016 Nov 30;13(1):199. doi: 10.1186/s12985-016-0658-4. Virol J. 2016. PMID: 27903277 Free PMC article. - Function and regulation of self-reactive marginal zone B cells in autoimmune arthritis.
Palm AK, Friedrich HC, Mezger A, Salomonsson M, Myers LK, Kleinau S. Palm AK, et al. Cell Mol Immunol. 2015 Jul;12(4):493-504. doi: 10.1038/cmi.2015.37. Epub 2015 May 11. Cell Mol Immunol. 2015. PMID: 25958842 Free PMC article.
References
- Ravetch JV, Kinet J-P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. - PubMed
- Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. - PubMed
- Fridman WH, Bonnerot C, Daëron M, Amigorena S, Teillaud JL, Sautes C. Structural bases of Fcγ receptor functions. Immunol Rev. 1992;125:49–76. - PubMed
- van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. - PubMed
- Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases