Blockade of a retinal cGMP-gated channel by polyamines - PubMed (original) (raw)
Blockade of a retinal cGMP-gated channel by polyamines
Z Lu et al. J Gen Physiol. 1999 Jan.
Abstract
The cyclic nucleotide-gated (CNG) channel in retinal rods converts the light-regulated intracellular cGMP concentration to various levels of membrane potential. Blockade of the channel by cations such as Ca2+ and Mg2+ lowers its effective conductance. Consequently, the membrane potential has very low noise, which enables rods to detect light with extremely high sensitivity. Here, we report that three polyamines (putrescine, spermidine, and spermine), which exist in both the intracellular and extracellular media, also effectively block the CNG channel from both sides of the membrane. Among them, spermine has the greatest potency. Extracellular spermine blocks the channel as a permeant blocker, whereas intracellular spermine appears to block the channel in two conformations-one permeant, and the other non- (or much less) permeant. The membrane potential in rods is typically depolarized to approximately -40 mV in the dark. At this voltage, K1/2 of the CNG channel for extracellular spermine is 3 microM, which is 100-1,000-fold higher affinity than that of the NMDA receptor-channel for extracellular spermine. Blockade of the CNG channel by polyamines may play an important role in suppressing noise in the signal transduction system in rods.
Figures
Figure 1
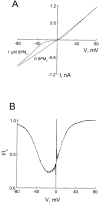
Blockade of the CNG channel by extracellular spermine. (A) Macroscopic current–voltage relations (I–V curves) of the CNG channel in the absence and presence of 1 μM extracellular spermine. (B) The fraction of unblocked current (I/Io), taken from A, is plotted against membrane voltage.
Figure 2
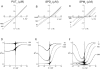
Comparison of CNG channel blockade by three extracellular polyamines. (A–C) Macroscopic I–V curves of the CNG channel in the absence and presence of various concentrations of extracellular putrescine (PUT), spermidine (SPD), and spermine (SPM). (D–F) The corresponding fraction of unblocked currents is plotted against membrane voltage.
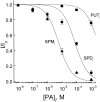
Figure 3
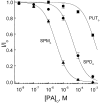
Comparative potency of the three extracellular polyamines. The fraction of unblocked currents at −40 mV is plotted against the concentrations of putrescine, spermidine, and spermine. The solid curves are least-squares fits of equations of the form I/Io = PA K 1/2/(PA K 1/2 + [PA]), where PA K 1/2 is the concentration at which a given polyamine blocks half the current through the CNG channel, and [PA] is the concentration of the polyamine. The K 1/2 values for extracellular putrescine, spermidine, and spermine are 1.4 mM, 50 μM, and 3.1 μM, respectively.
Figure 4
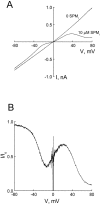
Blockade of the CNG channel by 10 μM intracellular spermine. (A) Macroscopic I–V curves of the CNG channel in the absence and presence of spermine. (B) The fraction of unblocked current, taken from A, is plotted against membrane voltage.
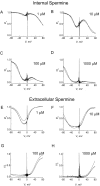
Figure 5
CNG channel blockade by three intracellular polyamines. (A–C) Macroscopic I–V curves of the CNG channel in the absence and the presence of various concentrations of intracellular putrescine, spermidine, and spermine. (D–F) The fraction of unblocked currents, taken from A–C, is plotted against membrane voltage.
Figure 6
Comparative potency of the three intracellular polyamines. The fraction of unblocked currents at +40 mV is plotted against the concentrations of putrescine, spermidine, and spermine. The solid curves are least-squares fits of equations of the forms I/Io = PA K 1/2/(PA K 1/2 + [PA]); see Fig. 3. The K 1/2 values for putrescine, spermidine, and spermine are 3.0 mM, 80 μM, and 6.7 μM, respectively.
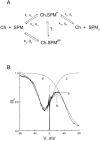
Figure 7
A kinetic model for the action of spermine. (A) Reaction scheme. Ch represents the CNG channel, SPMi and SPMo denote intra- and extracellular spermine, and Ch.SPMP and Ch.SPMNP denote the CNG channels blocked by spermine in the permeant and nonpermeant conformations, respectively. Kx and Qx are, respectively, the equilibrium dissociation constant at 0 mV and the total number of equivalent charges moving across the electrical field (in either direction) for nonpermeant spermine binding. Rate constants at 0 mV (kx or k−x) and the number of equivalent charges traversing the electrical field (qx or q−x) for movements of the permeant conformation of spermine are also shown. (The number of equivalent charges is defined as the actual number of charges of the blocker multiplied by the fraction of the electrical field transversed by the blocker.) (B) Noisy trace a is the same as that in Fig. 4 B, obtained in the presence of 10 μM of intracellular spermine. Curve b was drawn according to the scheme in A, with k1 = 1 * 106 M−1 s−1, k−1 = 8 s−1, k2 = 1.3 * 106 M−1 s−1, k−2 = 10.4 s−1, K3 = 333 μM, q1 = 2, q−1 = 0, q2 = 2, q−2 = 0, Q 3 = 1.5. (Any set of four rate constants in the given proportion will yield the same result.) Intracellular and extracellular spermine concentrations were set at 10 μM and zero, respectively. Curves c and d were drawn using the same parameters, except that both k1 and k−1 were zero for curve c and that K3 was infinite for curve d.
Figure 8
Modeling of channel blockade by various concentrations of intracellular and extracellular spermine. The experimental data (noisy traces) are the same as those in Figs. 2 F and 5 F. All smooth traces were computed according to the scheme in Fig. 7 A, using the same parameters as in Fig. 7 B and appropriate concentrations of intracellular and extracellular spermine. For example, in Fig. 8 A the model's intracellular spermine concentration was set at 1 μM and extracellular spermine concentration at zero.
Similar articles
- Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells.
Miller JL, Korenbrot JI. Miller JL, et al. J Gen Physiol. 1994 Nov;104(5):909-40. doi: 10.1085/jgp.104.5.909. J Gen Physiol. 1994. PMID: 7876828 Free PMC article. - Mechanism of cGMP-gated channel block by intracellular polyamines.
Guo D, Lu Z. Guo D, et al. J Gen Physiol. 2000 Jun;115(6):783-98. doi: 10.1085/jgp.115.6.783. J Gen Physiol. 2000. PMID: 10828251 Free PMC article. - Divalent cation selectivity is a function of gating in native and recombinant cyclic nucleotide-gated ion channels from retinal photoreceptors.
Hackos DH, Korenbrot JI. Hackos DH, et al. J Gen Physiol. 1999 Jun;113(6):799-818. doi: 10.1085/jgp.113.6.799. J Gen Physiol. 1999. PMID: 10352032 Free PMC article. - Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.
Korenbrot JI, Rebrik TI. Korenbrot JI, et al. Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review. - Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates.
Korenbrot JI. Korenbrot JI. Cell Calcium. 1995 Oct;18(4):285-300. doi: 10.1016/0143-4160(95)90025-x. Cell Calcium. 1995. PMID: 8556768 Review.
Cited by
- Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel.
Huang CJ, Moczydlowski E. Huang CJ, et al. Biophys J. 2001 Mar;80(3):1262-79. doi: 10.1016/S0006-3495(01)76102-4. Biophys J. 2001. PMID: 11222290 Free PMC article. - Monovalent cation (MC) current in cardiac and smooth muscle cells: regulation by intracellular Mg2+ and inhibition by polycations.
Zakharov SI, Smani T, Leno E, Macianskiene R, Mubagwa K, Bolotina VM. Zakharov SI, et al. Br J Pharmacol. 2003 Jan;138(1):234-44. doi: 10.1038/sj.bjp.0705074. Br J Pharmacol. 2003. PMID: 12522095 Free PMC article. - Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins.
Bowie D. Bowie D. J Biol Chem. 2018 Nov 30;293(48):18789-18802. doi: 10.1074/jbc.TM118.003794. Epub 2018 Oct 17. J Biol Chem. 2018. PMID: 30333231 Free PMC article. Review. - The role of polyamine metabolism in cellular function and physiology.
Schibalski RS, Shulha AS, Tsao BP, Palygin O, Ilatovskaya DV. Schibalski RS, et al. Am J Physiol Cell Physiol. 2024 Aug 1;327(2):C341-C356. doi: 10.1152/ajpcell.00074.2024. Epub 2024 Jun 17. Am J Physiol Cell Physiol. 2024. PMID: 38881422 Review. - Intracellular Spermine Is a Key Player in GSG1L's Regulation of Calcium-Permeable AMPAR Channel Conductance and Recovery from Desensitization.
McGee TP, Bats C, Farrant M, Cull-Candy SG. McGee TP, et al. J Neurosci. 2025 May 7;45(19):e1930242025. doi: 10.1523/JNEUROSCI.1930-24.2025. J Neurosci. 2025. PMID: 40185633
References
- Araneda RC, Zukin RS, Bennett MVL. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: a whole-cell and single channel study. Neurosci Lett. 1993;152:107–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous