The BCR-ABL oncoprotein potentially interacts with the xeroderma pigmentosum group B protein - PubMed (original) (raw)
The BCR-ABL oncoprotein potentially interacts with the xeroderma pigmentosum group B protein
N Takeda et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The previously uncharacterized CDC24 homology domain of BCR, which is missing in the P185 BCR-ABL oncogene of Philadelphia chromosome (Ph1)-positive acute lymphocytic leukemia but is retained in P210 BCR-ABL of chronic myelogeneous leukemia, was found to bind to the xeroderma pigmentosum group B protein (XPB). The binding appeared to be required for XPB to be tyrosine-phosphorylated by BCR-ABL. The interaction not only reduced both the ATPase and the helicase activities of XPB purified in the baculovirus system but also impaired XPB-mediated cross-complementation of the repair deficiency in rodent UV-sensitive mutants of group 3. The persistent dysfunction of XPB may in part underlie genomic instability in blastic crisis.
Figures
Figure 1
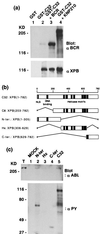
Expression of GST-C32 and BCR-ABL in 27–1 cells. GST-C32 was overexpressed alone or in combination with P210 or P185 BCR-ABL in 27–1 cells. Total cell lysates (a and b) or glutathione Sepharose-bound preparations (c_–_f) from cells expressing GST (vector alone) (lane 1), GST-C32 (lane 2), GST-C32/P185 BCR-ABL (lane 3), GST-C32/P210 BCR-ABL (lane 4), GST/P185 BCR-ABL (lane 5), and GST/P210 BCR-ABL (lane 6) were subjected to anti-ABL (a and c), anti-XPB (b and d), anti-p62 (e), and anti-phosphotyrosine (PY) (f) Western blotting.
Figure 2
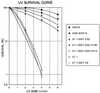
XPB cannot correct the nucleotide excision repair defect of 27–1 cells that express P210 BCR-ABL. Shown is a UV survival curve of 27–1 and parental CHO-9 cells with the expression of various constructs as indicated in the box (see Fig. 1).
Figure 3
(a) Tyrosine phosphorylation is not required for the XPB/P210 BCR-ABL interaction. Glutathione Sepharose-bound preparations from 27–1 cells overexpressing GST (lane 1), GST-C32 (lane 2), GST-C32 with BCR (lane 3), and GST-C32 with a kinase negative mutant of P210 BCR-ABL (KN P210) (lane 4) were subjected to anti-BCR (Upper) and anti-XPB (Lower) Western blotting. (b) Schematic representation of the isolated XPB clones (C8 and C32) and three XPB fragments (N-ter, Hx, and C-ter) constructed from C32. The putative nuclear localization signal (NLS), the potential DNA-binding domain, and conserved helicase motifs are indicated. The amino acid numbers are shown on the top. (c) XPB N-ter and C-ter fragments were tyrosine-phosphorylated by P210 BCR-ABL. _E. coli_-expressed and glutathione Sepharose-bound GST (lane 1, MOCK), GST-N-ter (lane 2), GST-Hx (lane 3), GST-C-ter (lane 4) as shown in b, and GST-C32 (lane 5) were incubated with total cell lysates from Sf9 cells infected with P210 BCR-ABL baculovirus (T) to allow binding. After washing, each sample was subjected to an in vitro kinase assay followed by anti-ABL (Upper) and anti-PY (Lower) Western blotting.
Figure 4
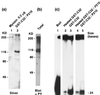
P210-bound and tyrosine-phosphorylated GST-C32 (GST-tagged full length XPB) expressed in Sf9 cells has reduced ATPase and helicase activities. (a) Purified myosin (0.2 μg) (lane 1) and GST-C32 bound to P210 BCR-ABL (lane 2) expressed in and purified from Sf9 cells were stained by silver nitrate. (b) Purified GST-C32 complexed to P210 shown in a was subjected to anti-PY Western blotting. Note a high level of tyrosine phosphorylation in GST-C32 comparable to that of autophosphorylated P210 BCR-ABL. (c) Direction-specific helicase substrates (see Materials and Methods) were boiled for 2 min (positive control, lane 1), were treated with purified GST-C32 after (lane 2) or before (lane 3) heat inactivation or with purified GST-C32 complexed to P210 BCR-ABL after (lane 4) or before (lane 5) heat inactivation, and were run on a 10% polyacrylamide gel.
Figure 5
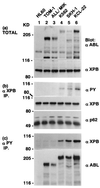
XPB is tyrosine-phosphorylated and possibly forms a complex with P210 BCR-ABL in CML cells. Total cell lysates (a), anti-XPB immunoprecipitates (b), and anti-phosphotyrosine immunoprecipitates (c) from human leukemic cell lines HL60 (derived from Ph1-negative acute promyelocytic leukemia) (lane 1), two cell lines from Ph1-positive ALL [TOM-1 (lane 2) and ALL/MIK (lane 3)] (28), three cell lines from Ph1-positive CML in blastic crisis [K562 (lane 4), SKH-1 (lane 5), and KCL-22 (lane 6)] (7) were subjected to anti-ABL (a, Upper and c, Lower), anti-XPB (a, Upper, b, Middle, and c, Upper), anti-PY (b, Top), and anti-p62 (b, Bottom) Western blot analyses.
Similar articles
- BCR binds to the xeroderma pigmentosum group B protein.
Maru Y, Kobayashi T, Tanaka K, Shibuya M. Maru Y, et al. Biochem Biophys Res Commun. 1999 Jul 5;260(2):309-12. doi: 10.1006/bbrc.1999.0822. Biochem Biophys Res Commun. 1999. PMID: 10403766 - FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia.
Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. Neviani P, et al. J Clin Invest. 2007 Sep;117(9):2408-21. doi: 10.1172/JCI31095. J Clin Invest. 2007. PMID: 17717597 Free PMC article. - TFIIH functions are altered by the P210BCR-ABL oncoprotein produced on the Philadelphia chromosome.
Maru Y, Bergmann E, Coin F, Egly JM, Shibuya M. Maru Y, et al. Mutat Res. 2001 Nov 1;483(1-2):83-8. doi: 10.1016/s0027-5107(01)00229-9. Mutat Res. 2001. PMID: 11600136 - Bcr: a negative regulator of the Bcr-Abl oncoprotein in leukemia.
Arlinghaus RB. Arlinghaus RB. Oncogene. 2002 Dec 9;21(56):8560-7. doi: 10.1038/sj.onc.1206083. Oncogene. 2002. PMID: 12476302 Review. - A coiled-coil tetramerization domain of BCR-ABL is essential for the interactions of SH2-containing signal transduction molecules.
Tauchi T, Miyazawa K, Ohyashiki K, Toyama K. Tauchi T, et al. Hum Cell. 1996 Dec;9(4):333-6. Hum Cell. 1996. PMID: 9183666 Review.
Cited by
- Secondary Resistant Mutations to Small Molecule Inhibitors in Cancer Cells.
Hamid AB, Petreaca RC. Hamid AB, et al. Cancers (Basel). 2020 Apr 9;12(4):927. doi: 10.3390/cancers12040927. Cancers (Basel). 2020. PMID: 32283832 Free PMC article. Review. - Molecular biology of chronic myeloid leukemia.
Maru Y. Maru Y. Int J Hematol. 2001 Apr;73(3):308-22. doi: 10.1007/BF02981955. Int J Hematol. 2001. PMID: 11345196 Review. - XPB induces C1D expression to counteract UV-induced apoptosis.
Li G, Liu J, Abu-Asab M, Masabumi S, Maru Y. Li G, et al. Mol Cancer Res. 2010 Jun;8(6):885-95. doi: 10.1158/1541-7786.MCR-09-0467. Epub 2010 Jun 8. Mol Cancer Res. 2010. PMID: 20530579 Free PMC article. - BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia.
Burke BA, Carroll M. Burke BA, et al. Leukemia. 2010 Jun;24(6):1105-12. doi: 10.1038/leu.2010.67. Epub 2010 May 6. Leukemia. 2010. PMID: 20445577 Free PMC article. Review. - Stem cell and kinase activity-independent pathway in resistance of leukaemia to BCR-ABL kinase inhibitors.
Li S, Li D. Li S, et al. J Cell Mol Med. 2007 Nov-Dec;11(6):1251-62. doi: 10.1111/j.1582-4934.2007.00108.x. J Cell Mol Med. 2007. PMID: 18205699 Free PMC article. Review.
References
- Maru Y, Witte O N. In: Appplication of Basic Science to Hematopoiesis and Treatment of Disease. Thomas E D, editor. New York: Raven; 1993. pp. 123–143.
- Champlin R E, Golde D W. Blood. 1985;65:1039–1047. - PubMed
- Towatari M, Adachi K, Kato H, Saito H. Blood. 1991;78:2178–2181. - PubMed
- Sill H, Goldman J M, Cross N C P. Blood. 1995;85:2013–2016. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous