Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage - PubMed (original) (raw)
Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage
C P Taylor et al. J Neurosci. 1999.
Abstract
Effects of oxygen/glucose deprivation (OGD) on subcellular elemental composition and water content were determined in nerve cell bodies from CA1 areas of rat hippocampal slices. Electron probe x-ray microanalysis was used to measure percentage water and concentrations of Na, P, K, Cl, Mg, and Ca in cytoplasm, nucleus, and mitochondria of cells exposed to normal and oxygen/glucose deficient medium. As an early (2 min) consequence of OGD, evoked synaptic potentials were lost, and K, Cl, P, and Mg concentrations decreased significantly in all morphological compartments. As exposure to in vitro OGD continued, a negative DC shift in interstitial voltage occurred ( approximately 5 min), whereas general elemental disruption worsened in cytoplasm and nucleus (5-42 min). Similar elemental changes were noted in mitochondria, except that Ca levels increased during the first 5 min of OGD and then decreased over the remaining experimental period (12-42 min). Compartmental water content decreased early (2 min), returned to control after 12 min of OGD, and then exceeded control levels at 42 min. After OGD (12 min), perfusion of hippocampal slices with control oxygenated solutions (reoxygenation) for 30 min did not restore synaptic function or improve disrupted elemental composition. Notably, reoxygenated CA1 cell compartments exhibited significantly elevated Ca levels relative to those associated with 42 min of OGD. When slices were incubated at 31 degreesC (hypothermia) during OGD/reoxygenation, neuronal dysfunction and elemental deregulation were minimal. Results show that in vitro OGD causes loss of transmembrane Na, K, and Ca gradients in CA1 neurons of hippocampal slices and that hypothermia can obtund this damaging process and preserve neuronal function.
Figures
Fig. 1.
Hematoxylin and eosin-stained sections (3-μm-thick) illustrating changes in slice morphology as a function of different incubation conditions. A, Tissue fixed immediately after slicing (nonincubated). Note swollen pyramidal cell nuclei and dendritic shafts, as well as pronounced blebbing (granular appearance) in dendritic areas. B, Slice fixed after ∼1.5 hr of incubation in normal aCSF at 36°C. Neuronal perikarya, nuclei, and apical dendrites exhibit nearly normal appearance.C, Tissue fixed after incubation for 1.5 hr in normal aCSF at 36°C followed by 12 min ischemia and 30 min reperfusion. Grossly swollen CA1 neuronal cell bodies and nuclei were observed with some disintegrated somata and others that stained darkly (pyknosis). Dendritic areas are highly granular, and individual dendritic shafts cannot be distinguished. D, Tissue fixed after incubation for 1.5 hr in normal aCSF at 31°C followed by 12 min ischemia and 30 min reperfusion. Preservation of histological features is remarkable and is similar in appearance to hippocampal regions of brain fixed in situ by cardiac perfusion (data not shown). Scale bar, 25 μm (for all micrographs).
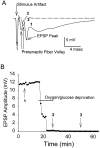
Fig. 2.
Evoked EPSPs were recorded at 1 min intervals throughout a representative OGD experiment (36°C). A, EPSP waveform evoked by 0.4 msec stimulus of Shaffer collaterals before OGD (1; solid trace), loss of EPSP and presynaptic fiber volley at end of OGD (2; dotted trace), and recovery of fiber volley (but not EPSP) 20 min after OGD (3; dashed trace).B, The maximal EPSP amplitude was plotted throughout a representative experiment. In this example, EPSPs were irreversibly lost after 12 min of OGD. Numerals refer to individual traces from A. Note that with lowered temperature, most slices recovered EPSPs after OGD (Table 1).
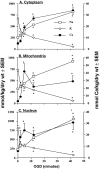
Fig. 3.
Mean (±SEM) dry weight Na, K, and Ca concentrations (millimoles of element per kilogram) in cytoplasm (A3), mitochondria (B3), and nucleus (C3) of CA1 nerve cells from rat hippocampal slices. Slices were exposed to oxygen/glucose-deficient aCSF for 2, 5, 12, or 42 min. Zero (0) time = pooled control data as presented in Table2. Number of analyses per compartment is presented in Table 4. Left ordinate is concentration scale for Na and K; right ordinate is the Ca scale. * indicates significantly different (p < 0.05) from time (0) control.
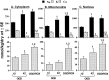
Fig. 4.
Mean (± SEM) dry weight Na, K, and Ca concentrations (millimoles of element per kilogram) in cytoplasm (A4), mitochondria (B4), and nucleus (C4) of CA1 nerve cells from rat hippocampal slices. Slices were exposed to oxygen/glucose-deficient aCSF for 12 or 42 min (OGD). To measure changes in cellular elemental composition during reoxygenation (ROX), hippocampal slices were exposed to OGD for 12 min followed by 30 min of perfusion with normal oxygenated aCSF (OGD/ROX).C, Pooled control data as presented in Table 2.Top graphs present Na and K data; bottom graphs present Ca data. 1 indicates significantly different (p < 0.05) from 12 min (12′) OGD data; 2 indicates significantly different (p < 0.05) from 42 min (42′) OGD data.
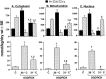
Fig. 5.
Mean (±SEM) dry weight Na, K, and Ca concentrations (millimoles of element per kilogram) in cytoplasm (5A), mitochondria (5B), and nucleus (5C) of CA1 nerve cells from rat hippocampal slices. Slices were exposed to oxygen/glucose-deficient aCSF for 12 min followed by incubation with normal medium for 30 min (OGD/ROX). To assess the potential neuroprotective effect of hypothermia, aCSF temperature was maintained at either 36°C or 31°C throughout the ischemic and post-ischemic periods. C, Pooled control data as presented in Table 2. Number of analyses per compartment is presented in Table 4. Top graphs present Na and K data; _bottom graphs_present Ca data. 1 indicates significantly different (p < 0.05) from control (C); 2 indicates significantly different (p < 0.05) from reoxygenation at 36°C.
Similar articles
- Effects of ion channel blockade on the distribution of Na, K, Ca and other elements in oxygen-glucose deprived CA1 hippocampal neurons.
LoPachin RM, Gaughan CL, Lehning EJ, Weber ML, Taylor CP. LoPachin RM, et al. Neuroscience. 2001;103(4):971-83. doi: 10.1016/s0306-4522(01)00035-5. Neuroscience. 2001. PMID: 11301205 - Elemental composition and water content of rat optic nerve myelinated axons during in vitro post-anoxia reoxygenation.
Stys PK, Lopachin RM Jr. Stys PK, et al. Neuroscience. 1996 Aug;73(4):1081-90. doi: 10.1016/0306-4522(96)00114-5. Neuroscience. 1996. PMID: 8809826 - The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices.
Pugliese AM, Traini C, Cipriani S, Gianfriddo M, Mello T, Giovannini MG, Galli A, Pedata F. Pugliese AM, et al. Br J Pharmacol. 2009 Jul;157(5):818-30. doi: 10.1111/j.1476-5381.2009.00218.x. Epub 2009 May 5. Br J Pharmacol. 2009. PMID: 19422385 Free PMC article. - Chloride transport inhibitors influence recovery from oxygen-glucose deprivation-induced cellular injury in adult hippocampus.
Pond BB, Galeffi F, Ahrens R, Schwartz-Bloom RD. Pond BB, et al. Neuropharmacology. 2004 Aug;47(2):253-62. doi: 10.1016/j.neuropharm.2004.04.002. Neuropharmacology. 2004. PMID: 15223304 - [Relation between GLu-R and the protective effect of hypothermia on oxygen and glucose deprivation injury in hippocampal slice or rat].
Liu SL, Wang ZP, Zeng YM, Jiang S, Wang SQ. Liu SL, et al. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2005 May;21(2):127-32. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2005. PMID: 21171321 Chinese.
Cited by
- Single-cell resolution mapping of neuronal damage in acute focal cerebral ischemia using thallium autometallography.
Stöber F, Baldauf K, Ziabreva I, Harhausen D, Zille M, Neubert J, Reymann KG, Scheich H, Dirnagl U, Schröder UH, Wunder A, Goldschmidt J. Stöber F, et al. J Cereb Blood Flow Metab. 2014 Jan;34(1):144-52. doi: 10.1038/jcbfm.2013.177. Epub 2013 Oct 16. J Cereb Blood Flow Metab. 2014. PMID: 24129748 Free PMC article. - Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it?
Chao D, Xia Y. Chao D, et al. Prog Neurobiol. 2010 Apr;90(4):439-70. doi: 10.1016/j.pneurobio.2009.12.007. Epub 2009 Dec 28. Prog Neurobiol. 2010. PMID: 20036308 Free PMC article. Review. - Blockade of soluble epoxide hydrolase attenuates post-ischemic neuronal hyperexcitation and confers resilience against stroke with TrkB activation.
Chang LH, Lin HC, Huang SS, Chen IC, Chu KW, Chih CL, Liang YW, Lee YC, Chen YY, Lee YH, Lee IH. Chang LH, et al. Sci Rep. 2018 Jan 8;8(1):118. doi: 10.1038/s41598-017-18558-6. Sci Rep. 2018. PMID: 29311641 Free PMC article. - Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors.
Zhou M, Baudry M. Zhou M, et al. J Neurosci. 2006 Mar 15;26(11):2956-63. doi: 10.1523/JNEUROSCI.4299-05.2006. J Neurosci. 2006. PMID: 16540573 Free PMC article. - Dose enhancement effects to the nucleus and mitochondria from gold nanoparticles in the cytosol.
McNamara AL, Kam WW, Scales N, McMahon SJ, Bennett JW, Byrne HL, Schuemann J, Paganetti H, Banati R, Kuncic Z. McNamara AL, et al. Phys Med Biol. 2016 Aug 21;61(16):5993-6010. doi: 10.1088/0031-9155/61/16/5993. Epub 2016 Jul 20. Phys Med Biol. 2016. PMID: 27435339 Free PMC article.
References
- Almeida A, Allen KL, Bates TE, Clark JB. Effect of reperfusion following cerebral ischaemia on the activity of the mitochondrial respiratory chain in the gerbil brain. J Neurochem. 1995;65:1698–1703. - PubMed
- Alvarez-Leefmans FJ, Giraldez F, Gamino SM. Intracellular free magnesium in excitable cells: its measurement and its biologic significance. Can J Physiol Pharmacol. 1987;65:915–925. - PubMed
- Andersen P, Lomo T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp Brain Res. 1966;2:247–260. - PubMed
- Balestrino M, Aitken PG, Somjen GG (1989) Spreading depression-like hypoxic depolarization in CA 1 and fascia dentata of hippocampal slices: relationship to selective vulnerability. Brain Res 102–107. - PubMed
- Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore: a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous