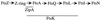Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL - PubMed (original) (raw)
Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL
D S Weiss et al. J Bacteriol. 1999 Jan.
Abstract
Assembly of the division septum in bacteria is mediated by several proteins that localize to the division site. One of these, FtsI (also called penicillin-binding protein 3) of Escherichia coli, consists of a short cytoplasmic domain, a single membrane-spanning segment, and a large periplasmic domain that encodes a transpeptidase activity involved in synthesis of septal peptidoglycan. We have constructed a merodiploid strain with a wild-type copy of ftsI at the normal chromosomal locus and a genetic fusion of ftsI to the green fluorescent protein (gfp) at the lambda attachment site. gfp-ftsI was expressed at physiologically appropriate levels under control of a regulatable promoter. Consistent with previous results based on immunofluorescence microscopy GFP-FtsI localized to the division site during the later stages of cell growth and throughout septation. Localization of GFP-FtsI to the cell pole(s) was not observed unless the protein was overproduced about 10-fold. Membrane anchor alterations shown previously to impair division but not membrane insertion or transpeptidase activity were found to interfere with localization of GFP-FtsI to the division site. In contrast, GFP-FtsI localized well in the presence of beta-lactam antibiotics that inhibit the transpeptidase activity of FtsI. Septal localization depended upon every other division protein tested (FtsZ, FtsA, FtsQ, and FtsL). We conclude that FtsI is a late recruit to the division site, and that its localization depends on an intact membrane anchor.
Figures
FIG. 1
Steady-state levels of FtsI and GFP-FtsI fusion proteins as determined by Western blotting with an anti-FtsI antibody. Molecular mass standards are indicated to the left of the blot, and the positions of FtsI and GFP-FtsI are shown to the right. The GFP fusion protein produced is indicated above each lane. The faint band at the position of GFP-FtsI in the first lane is due to cross-reaction of the primary antibody with a protein of unknown identity. The intensity of this band does not respond to IPTG. In contrast, the proteins identified as GFP-FtsI respond to IPTG and are also detected with anti-GFP antibodies (not shown). The strains used were MC4100, EC436, EC505, EC507, EC509, and EC511.
FIG. 2
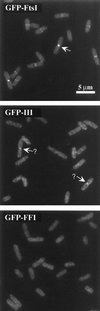
Localization of GFP-FtsI, GFP-III, and GFP-FFI in cells fixed during exponential growth. Arrows indicate examples of FtsI rings at division sites. These are very faint in the case of GFP-III and not detected in GFP-FFI. In the case of GFP-FtsI, 10 of the 13 cells shown were judged to have an FtsI ring at the division site. The strains used were EC436, EC505, and EC507.
FIG. 3
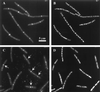
Localization of FtsI in filaments formed by treating cells with FtsI-specific β-lactam antibiotics. Upper panels show strain EC522, expressing gfp-ftsI, after 75 min in the presence of cephalexin. (A) GFP. (B) DAPI. Lower panels show FtsI detected by IFM of strain MC4100 after 45 min in the presence of furazlocillin. Arrows indicate FtsI localized to potential division sites. (C) Fluorescein. (D) Propidium iodide.
FIG. 4
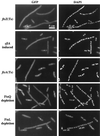
Dependence of localization of GFP-FtsI on FtsZ, FtsA, FtsQ, and FtsL. In each case, one or more filaments is shown together with nonfilamenting cells that do exhibit GFP-FtsI bands and serve as internal controls for microscopy and subsequent image processing. These latter are the short cells in the micrographs. Filaments are as follows: EC458 [ftsZ(Ts)] after 60 min at 42°C; EC436/pDSW259 (sfiA induced) 60 min after addition of arabinose; EC455 [ftsA(Ts)] after 45 min at 42°C; EC538 (FtsQ depletion) in glucose; EC607 (FtsL depletion) in glucose. Controls were EC436 after 60 min at 42°C, EC436/pDSW259 60 min after addition of glucose, EC436 after 45 min at 42°C, EC538 in arabinose, and EC607 in arabinose.
FIG. 5
Localization of ZipA-GFP in an ftsI23(Ts) mutant background (EC491). Cells were grown at 30°C prior to fixation (A) and were shifted to 42°C for 60 min prior to fixation (B).
FIG. 6
Dependency relationships for septal localization of several E. coli division proteins. The first event is polymerization of FtsZ into the Z ring at the future division sites. The other proteins then localize to that site in the order indicated. The positions of ZipA, FtsK, and FtsW are only partially established. Unpublished work from our lab indicates that ZipA localization requires a Z ring but not FtsA. FtsK requires FtsA but not FtsI. FtsW is known to localize, but what happens in fts mutant backgrounds is not known. The model is based on our own results in this paper, references , , , and , and reports from several other labs (2, 3, 5, 38, 53, 59).
Similar articles
- Localization of FtsL to the Escherichia coli septal ring.
Ghigo JM, Weiss DS, Chen JC, Yarrow JC, Beckwith J. Ghigo JM, et al. Mol Microbiol. 1999 Jan;31(2):725-37. doi: 10.1046/j.1365-2958.1999.01213.x. Mol Microbiol. 1999. PMID: 10027987 - FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division.
Chen JC, Beckwith J. Chen JC, et al. Mol Microbiol. 2001 Oct;42(2):395-413. doi: 10.1046/j.1365-2958.2001.02640.x. Mol Microbiol. 2001. PMID: 11703663 - The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site.
Mercer KL, Weiss DS. Mercer KL, et al. J Bacteriol. 2002 Feb;184(4):904-12. doi: 10.1128/jb.184.4.904-912.2002. J Bacteriol. 2002. PMID: 11807049 Free PMC article. - Cytokinesis in bacteria.
Errington J, Daniel RA, Scheffers DJ. Errington J, et al. Microbiol Mol Biol Rev. 2003 Mar;67(1):52-65, table of contents. doi: 10.1128/MMBR.67.1.52-65.2003. Microbiol Mol Biol Rev. 2003. PMID: 12626683 Free PMC article. Review. - The structure and function of Escherichia coli penicillin-binding protein 3.
Nguyen-Distèche M, Fraipont C, Buddelmeijer N, Nanninga N. Nguyen-Distèche M, et al. Cell Mol Life Sci. 1998 Apr;54(4):309-16. doi: 10.1007/s000180050157. Cell Mol Life Sci. 1998. PMID: 9614966 Free PMC article. Review.
Cited by
- A mutation in Escherichia coli ftsZ bypasses the requirement for the essential division gene zipA and confers resistance to FtsZ assembly inhibitors by stabilizing protofilament bundling.
Haeusser DP, Rowlett VW, Margolin W. Haeusser DP, et al. Mol Microbiol. 2015 Sep;97(5):988-1005. doi: 10.1111/mmi.13081. Epub 2015 Jul 4. Mol Microbiol. 2015. PMID: 26046682 Free PMC article. - Inhibiting cell division in Escherichia coli has little if any effect on gene expression.
Arends SJ, Weiss DS. Arends SJ, et al. J Bacteriol. 2004 Feb;186(3):880-4. doi: 10.1128/JB.186.3.880-884.2004. J Bacteriol. 2004. PMID: 14729718 Free PMC article. - Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion.
Kamath S, Chen ML, Chakrabarty AM. Kamath S, et al. J Bacteriol. 2000 Jul;182(13):3826-31. doi: 10.1128/JB.182.13.3826-3831.2000. J Bacteriol. 2000. PMID: 10851000 Free PMC article. - Streptococcus mutans yidC1 and yidC2 Impact Cell Envelope Biogenesis, the Biofilm Matrix, and Biofilm Biophysical Properties.
Palmer SR, Ren Z, Hwang G, Liu Y, Combs A, Söderström B, Lara Vasquez P, Khosravi Y, Brady LJ, Koo H, Stoodley P. Palmer SR, et al. J Bacteriol. 2018 Dec 7;201(1):e00396-18. doi: 10.1128/JB.00396-18. Print 2019 Jan 1. J Bacteriol. 2018. PMID: 30322852 Free PMC article. - The C-terminal tail of the bacterial translocation ATPase SecA modulates its activity.
Jamshad M, Knowles TJ, White SA, Ward DG, Mohammed F, Rahman KF, Wynne M, Hughes GW, Kramer G, Bukau B, Huber D. Jamshad M, et al. Elife. 2019 Jun 27;8:e48385. doi: 10.7554/eLife.48385. Elife. 2019. PMID: 31246174 Free PMC article.
References
- Adam M, Fraipont C, Rhazi N, Nguyen-Distèche M, Lakaye B, Frère J M, Devreese B, Van Beeumen J, van Heijenoort Y, van Heijenoort J, Ghuysen J M. The bimodular G57-V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyzes peptide bond formation from thiolesters and does not catalyze glycan chain polymerization from the lipid II intermediate. J Bacteriol. 1997;179:6005–6009. - PMC - PubMed
- Addinall S G, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Mol Microbiol. 1997;25:303–309. - PubMed
- Addinall S G, Cao C, Lutkenhaus J. Temperature shift experiments with an ftsZ84(Ts) strain reveal rapid dynamics of FtsZ localization and indicate that the Z ring is required throughout septation and cannot reoccupy division sites once constriction has initiated. J Bacteriol. 1997;179:4277–4284. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases