Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice - PubMed (original) (raw)
Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice
J L Clements et al. J Clin Invest. 1999 Jan.
Abstract
The adapter protein SLP-76 is expressed in T lymphocytes and hematopoietic cells of the myeloid lineage, and is known to be a substrate of the protein tyrosine kinases that are activated after ligation of the T-cell antigen receptor. Transient overexpression of SLP-76 in a T-cell line potentiates transcriptional activation after T-cell receptor ligation, while loss of SLP-76 expression abrogates several T-cell receptor-dependent signaling pathways. Mutant mice that lack SLP-76 manifest a severe block at an early stage of thymocyte development, implicating SLP-76 in signaling events that promote thymocyte maturation. While it is clear that SLP-76 plays a key role in development and activation of T lymphocytes, relatively little is understood regarding its role in transducing signals initiated after receptor ligation in other hematopoietic cell types. In this report, we describe fetal hemorrhage and perinatal mortality in SLP-76-deficient mice. Although megakaryocyte and platelet development proceeds normally in the absence of SLP-76, collagen-induced platelet aggregation and granule release is markedly impaired. Furthermore, treatment of SLP-76-deficient platelets with collagen fails to elicit tyrosine phosphorylation of phospholipase C-gamma2 (PLC-gamma2), suggesting that SLP-76 functions upstream of PLC-gamma2 activation. These data provide one potential mechanism for the fetal hemorrhage observed in SLP-76-deficient mice and reveal that SLP-76 expression is required for optimal receptor-mediated signal transduction in platelets as well as T lymphocytes.
Figures
Figure 1
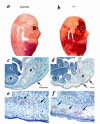
SLP-76–deficient mice manifest diffuse, subcutaneous hemorrhage and edema. SLP-76+/– mice were mated and day of gestation was calculated based on the presence of a vaginal plug. At approximately day 14 (a and b) or day 18 (c–f) of gestation, the mother was sacrificed and fetuses were isolated. Genotypes were determined by PCR analysis using genomic DNA isolated from a small tissue sample as template. (a and b) Gross morphological appearance of littermate control (a) or SLP-76–/– (b) E14 fetuses. (c–f) Histological appearance of SLP-76+/– (c and e) and SLP-76–/– (d and f) fetal sections at day 18 of gestation. (c and d) Caudal view of embryos. Note close association of epithelium with underlying connective tissue (arrow) in the SLP-76+/– fetus and edema and subcutaneous bleeding in the SLP-76–/– fetus. B, bone; G, gut; K, kidney; L, liver. Bar represents 1000 μM. (e and f) High-power view of subcutaneous region. Note intact endothelium and intraluminal nucleated red blood cells (arrows) in SLP-76+/– embryo compared with attenuated endothelium, extravasated blood (arrow), and subcutaneous edema in the SLP-76–/– embryo. Bar represents 200 μM.
Figure 2
Platelets and megakaryocytes develop normally and exhibit normal morphology in the absence of SLP-76. The humerus and peripheral blood were isolated from SLP-76+/+ (a–c) or SLP-76–/– (d–f) mice. Bone marrow sections (a and d), whole blood smears (b and e), or glutaraldehyde-fixed platelet sections were generated as described in Methods. (a and d) Hematoxylin and eosin–stained humeral bone marrow sections (×100). Megakaryocytes are indicated with arrows. Note that the density of megakaryocytes in sections obtained from SLP-76+/+ and SLP-76–/– mice was similar (1.1–1.15 megakaryocytes per oil immersion field). (b and e) Wright-Giemsa staining of whole blood smears (×250). Platelets are indicated with arrows. (c and f) Transmission electron microscopic analysis of glutaraldehyde-fixed platelets. Large arrows indicate α granules and small arrows denote dense granules.
Figure 3
SLP-76 is tyrosine-phosphorylated in response to collagen. Platelets were isolated from normal mice and left untreated (basal, B) or incubated with thrombin (T), collagen (C), or pervanadate (PV) for the times indicated. Platelets were then lysed and subjected to immunoprecipitation with a murine SLP-76–specific antibody. Immunoprecipitates were washed, resolved by SDS-PAGE, transferred to nitrocellulose, and then immunoblotted with the phosphotyrosine-specific antibody 4G10 (α-pTyr). The immunoblot shown in the top panel was stripped and reblotted with an SLP-76–specific antibody (SLP-76) to demonstrate equal amounts of immunoprecipitated SLP-76 in each lane. Identical results were obtained in a separate experiment.
Figure 4
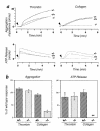
Platelet aggregation and ATP release in response to thrombin or collagen. Whole blood was isolated from SLP-76 +/+, +/–, or –/– mice, and platelet number was normalized before analysis. (a) Aggregation and ATP release tracings from one representative experiment. Arrowheads indicate the point at which the indicated agonist was added. (b) Change in impedence (AGGREGATION) or ATP concentration (ATP RELEASE) was determined for each sample at the same time point after addition of thrombin or collagen by measuring the amplitude of the response. The timepoint at which the response was measured correlated with the maximal response. The values obtained for the +/– and –/– samples were normalized by calculating the percent of the +/+ response. The SEM for each group is shown. _*_These data were obtained from four independent experiments. In all other cases, data was obtained from five independent experiments. _**_In all experiments, ATP release from SLP-76–/– platelets after exposure to collagen was not detectable.
Figure 5
Collagen induces tyrosine phosphorylation of Syk but not PLC-γ2 in SLP-76–deficient platelets. Platelets were isolated from SLP-76 +/+, +/–, or –/– mice and left resting (basal, B) or stimulated with collagen (C) for 90 s. Platelets were then lysed and subjected to immunoprecipitation with Syk-specific (top panels) or PLC-γ2–specific (bottom panels) antibodies. Immunoprecipitates were washed, resolved by SDS-PAGE, and then transferred to nitrocellulose. Filters were then immunoblotted with phosphotyrosine-specific antibody (α-pTyr). Filters were then stripped and reblotted with Syk- or PLC-γ2–specific antibodies to demonstrate equivalent sample loading.
Similar articles
- Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets.
Gross BS, Lee JR, Clements JL, Turner M, Tybulewicz VL, Findell PR, Koretzky GA, Watson SP. Gross BS, et al. J Biol Chem. 1999 Feb 26;274(9):5963-71. doi: 10.1074/jbc.274.9.5963. J Biol Chem. 1999. PMID: 10026222 - Role of Fc receptor gamma-chain in platelet glycoprotein Ib-mediated signaling.
Wu Y, Suzuki-Inoue K, Satoh K, Asazuma N, Yatomi Y, Berndt MC, Ozaki Y. Wu Y, et al. Blood. 2001 Jun 15;97(12):3836-45. doi: 10.1182/blood.v97.12.3836. Blood. 2001. PMID: 11389024 - BLNK: connecting Syk and Btk to calcium signals.
Kurosaki T, Tsukada S. Kurosaki T, et al. Immunity. 2000 Jan;12(1):1-5. doi: 10.1016/s1074-7613(00)80153-3. Immunity. 2000. PMID: 10661400 Review. No abstract available. - Regulation of phospholipase C-gamma2 and phosphoinositide 3-kinase pathways by adaptor proteins in B lymphocytes.
Kurosaki T, Okada T. Kurosaki T, et al. Int Rev Immunol. 2001;20(6):697-711. doi: 10.3109/08830180109045586. Int Rev Immunol. 2001. PMID: 11913946 Review.
Cited by
- Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge.
Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, Ratliff TL. Elzey BD, et al. Blood. 2008 Apr 1;111(7):3684-91. doi: 10.1182/blood-2007-05-091728. Epub 2008 Feb 6. Blood. 2008. PMID: 18256321 Free PMC article. - Coordination of receptor signaling in multiple hematopoietic cell lineages by the adaptor protein SLP-76.
Jordan MS, Koretzky GA. Jordan MS, et al. Cold Spring Harb Perspect Biol. 2010 Apr;2(4):a002501. doi: 10.1101/cshperspect.a002501. Epub 2010 Mar 17. Cold Spring Harb Perspect Biol. 2010. PMID: 20452948 Free PMC article. Review. - Modulation of T-cell receptor signal transduction by herpesvirus signaling adaptor protein.
Lee SH, Chung YH, Cho NH, Gwack Y, Feng P, Jung JU. Lee SH, et al. Mol Cell Biol. 2004 Jun;24(12):5369-82. doi: 10.1128/MCB.24.12.5369-5382.2004. Mol Cell Biol. 2004. PMID: 15169900 Free PMC article. - The N-terminal SH2 domain of Syk is required for (hem)ITAM, but not integrin, signaling in mouse platelets.
Hughes CE, Finney BA, Koentgen F, Lowe KL, Watson SP. Hughes CE, et al. Blood. 2015 Jan 1;125(1):144-54. doi: 10.1182/blood-2014-05-579375. Epub 2014 Oct 28. Blood. 2015. PMID: 25352128 Free PMC article. - Immune functions in mice lacking Clnk, an SLP-76-related adaptor expressed in a subset of immune cells.
Utting O, Sedgmen BJ, Watts TH, Shi X, Rottapel R, Iulianella A, Lohnes D, Veillette A. Utting O, et al. Mol Cell Biol. 2004 Jul;24(13):6067-75. doi: 10.1128/MCB.24.13.6067-6075.2004. Mol Cell Biol. 2004. PMID: 15199160 Free PMC article.
References
- Jackman J, et al. Molecular cloning of SLP-76, a 76 kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. - PubMed
- Fang N, Motto DG, Ross SE, Koretzky GA. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J Immunol. 1996;157:3769–3773. - PubMed
- Wardenburg JB, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. - PubMed
- Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases