Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases - PubMed (original) (raw)
Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases
D B Constam et al. J Cell Biol. 1999.
Abstract
Bone morphogenetic proteins (BMPs) are derived from inactive precursor proteins by endoproteolytic cleavage. Here we show that processing of Nodal and Myc-tagged BMP4 is significantly enhanced by SPC1/Furin or SPC4/PACE4, providing direct evidence that regulation of BMP signaling is likely to be controlled by subtilisin-like proprotein convertase (SPC) activities. Nodal processing is dramatically enhanced if two residues adjacent to the precursor cleavage site are substituted with amino acids found at the equivalent positions of Activin, demonstrating that structural constraints at the precursor cleavage site limit the processing efficiency. However, in transfection assays, mature Nodal is undetectable either in culture supernatants or in cell lysates, despite efficient cleavage of the precursor protein, suggesting that mature Nodal is highly unstable. Domain swap experiments support this conclusion since mature BMP4 or Dorsalin are also destabilized when expressed in conjunction with the Nodal pro domain. By contrast, mature Nodal is stabilized by the Dorsalin pro domain, which mediates the formation of stable complexes. Collectively, these data show that the half-life of mature BMPs is greatly influenced by the identity of their pro regions.
Figures
Figure 1
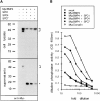
MycBMP4 precursor cleavage in COS-1 cells cotransfected with SPC1/furin, SPC4/PACE4, or SPC7. (A) Western blot analysis showing the presence of MycBMP4 precursor (bracket) in lysates (top) or supernatants (bottom) of cells transfected with 2 μg of MycBMP4 expression vector. In cell supernatants, mature MycBMP4 is detected at 23 kD (asterisk). Molecular masses of standard proteins are indicated to the left in kilodaltons. (B) Induction of alkaline phosphatase activity in C3H10T1/2 cells treated with serially diluted supernatants of transfected COS cells. The legend to the right shows the cDNA expression vectors used. SPC1 expression vector alone was used as a mock control, whereas MycDorsalin served as a positive control (Basler et al., 1993).
Figure 2
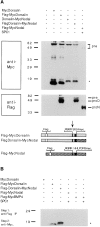
Nodal precursor cleavage is enhanced by SPC1 and SPC4, or by structural modification of the cleavage site. (A) Native Nodal or a mutant form, termed Nodal-H246L, was analyzed by Western blotting in cell lysates (top) or supernatants (bottom) using anti-Nodal antiserum directed against a peptide in the middle of the COOH-terminal domain. The mutant sequence in Nodal-H246L near the multibasic cleavage site is aligned with that of native Nodal (top). Where indicated, COS cells were cotransfected with 0.5 μg of either SPC1, SPC4, or SPC7 expression vector. Bands corresponding to unprocessed precursor are marked pre. Note the absence of any detectable mature Nodal. An additional, nonspecific band is present in all supernatants, suggesting that comparable amounts of protein were loaded. (B) Cleavage of Flag-tagged Nodal precursor and of FlagNodal-H246L visualized by anti-FLAG immunoblotting. The position of the Flag epitope (open box) relative to the cleavage site (arrow) within Nodal and Nodal-H246L is shown schematically. The Nodal pro domain (pro) accumulates in cell supernatants (bottom), but not in lysates (top). Cotransfection with SPC1 or SPC4, but not SPC7, resulted in enhanced precursor cleavage. (C) The multibasic motif R-Q-R-R present in Nodal-H246L is replaced by SQAG in the Flag-cmNodal-H246L construct, resulting in complete inhibition of processing by either resident COS cell proteases or by SPC1 transfection.
Figure 3
The Nodal pro region destabilizes mature MycBMP4. Expression of the chimeric protein Nodal-MycBMP4 in comparison with MycBMP4 analyzed in cell lysates (top left) or supernatants (SN, bottom left). The positions of precursor (bracket) and mature (asterisk) forms are indicated. Processed MycBMP4 accumulates in cell supernatants only if expressed in the context of its own pro domain, but not in association with the Nodal pro region. Flag-tagged pro BMP4 (proB4) and pro Nodal (proN) were detected in cell supernatants (top right), showing that Nodal-MycBMP4 is processed. The structures of the various precursors and the position of Flag (open boxes) and Myc epitopes (black boxes) relative to the precursor cleavage sites (arrow) are shown schematically.
Figure 4
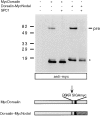
Mature Nodal is stabilized when expressed as a fusion protein with the Dorsalin pro region. MycDorsalin and Dorsalin-MycNodal fusion protein in COS cell supernatants analyzed by anti-Myc immunoblotting. Note that mature MycNodal (asterisk) is stable when expressed in conjunction with the Dorsalin pro region.
Figure 5
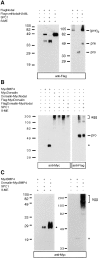
Mature MycNodal and mature MycDorsalin stably associate with the Dorsalin pro domain. (A) Insertion of a Flag epitope (open boxes) in their pro domains does not interfere with the processing of MycDorsalin, Dorsalin-MycNodal, or MycNodal (top), but allows detection of pro Dorsalin (proD) and pro Nodal (proN) fragments and unprocessed precursor (pre) proteins (bottom). (B) The supernatants shown in A were immunoprecipitated using anti-Flag antibodies and analyzed by anti-Myc immunoblotting. Mature MycDorsalin or MycNodal, respectively, coprecipitate with the Dorsalin pro region.
Figure 6
Formation of higher order BMP complexes. (A) Nodal proteins analyzed under reducing (left) or nonreducing conditions (right) by anti-Flag immunoblotting. Note the presence of a 100-kD protein corresponding in size to a dimer of unprocessed Nodal precursor, (pre)2, and of monomeric pro fragments (pro) in A and B. (B) MycBMP4, MycDorsalin, and Dorsalin-MycNodal analyzed in nonreducing SDS gels, followed by anti-Myc (left) or anti-Flag (right) immunoblotting. (C) Dorsalin pro region–mediated aggregation of mature Myc BMP4. agg, aggregates; asterisk, position of mature MycBMP4; β-ME, β-mercaptoethanol.
Similar articles
- Endoproteolytic processing of integrin pro-alpha subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7.
Lissitzky JC, Luis J, Munzer JS, Benjannet S, Parat F, Chrétien M, Marvaldi J, Seidah NG. Lissitzky JC, et al. Biochem J. 2000 Feb 15;346 Pt 1(Pt 1):133-8. Biochem J. 2000. PMID: 10657249 Free PMC article. - Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases.
Van de Ven WJ, Roebroek AJ, Van Duijnhoven HL. Van de Ven WJ, et al. Crit Rev Oncog. 1993;4(2):115-36. Crit Rev Oncog. 1993. PMID: 8420571 Review. - Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations.
Brakch N, Rist B, Beck-Sickinger AG, Goenaga J, Wittek R, Bürger E, Brunner HR, Grouzmann E. Brakch N, et al. Biochemistry. 1997 Dec 23;36(51):16309-20. doi: 10.1021/bi9714767. Biochemistry. 1997. PMID: 9405066 - The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4.
Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. Ben-Haim N, et al. Dev Cell. 2006 Sep;11(3):313-23. doi: 10.1016/j.devcel.2006.07.005. Dev Cell. 2006. PMID: 16950123 - The proprotein convertases furin and PACE4 play a significant role in tumor progression.
Bassi DE, Mahloogi H, Klein-Szanto AJ. Bassi DE, et al. Mol Carcinog. 2000 Jun;28(2):63-9. Mol Carcinog. 2000. PMID: 10900462 Review.
Cited by
- Production of Mature Recombinant Human Activin A in Transgenic Rice Cell Suspension Culture.
Do VG, Yang MS. Do VG, et al. Curr Issues Mol Biol. 2024 Jan 30;46(2):1164-1176. doi: 10.3390/cimb46020074. Curr Issues Mol Biol. 2024. PMID: 38392192 Free PMC article. - Internalization of proprotein convertase PC7 from plasma membrane is mediated by a novel motif.
Declercq J, Meulemans S, Plets E, Creemers JW. Declercq J, et al. J Biol Chem. 2012 Mar 16;287(12):9052-60. doi: 10.1074/jbc.M111.306407. Epub 2012 Jan 30. J Biol Chem. 2012. PMID: 22294700 Free PMC article. - Role of subtilisin-like convertases in cadherin processing or the conundrum to stall cadherin function by convertase inhibitors in cancer therapy.
Müller EJ, Caldelari R, Posthaus H. Müller EJ, et al. J Mol Histol. 2004 Mar;35(3):263-75. doi: 10.1023/b:hijo.0000032358.51866.a2. J Mol Histol. 2004. PMID: 15339046 Review. - Human disease-causing NOG missense mutations: effects on noggin secretion, dimer formation, and bone morphogenetic protein binding.
Marcelino J, Sciortino CM, Romero MF, Ulatowski LM, Ballock RT, Economides AN, Eimon PM, Harland RM, Warman ML. Marcelino J, et al. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11353-8. doi: 10.1073/pnas.201367598. Epub 2001 Sep 18. Proc Natl Acad Sci U S A. 2001. PMID: 11562478 Free PMC article. - A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni.
Freitas TC, Jung E, Pearce EJ. Freitas TC, et al. Int J Parasitol. 2009 Feb;39(3):281-7. doi: 10.1016/j.ijpara.2008.08.001. Epub 2008 Aug 15. Int J Parasitol. 2009. PMID: 18765241 Free PMC article.
References
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73:687–702. - PubMed
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. - PubMed
- Brennan SO, Nakayama K. Cleavage of proalbumin peptides by furin reveals unexpected restrictions at the P2 and P′1 sites. FEBS Lett. 1994;347:80–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases