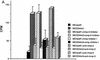Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion - PubMed (original) (raw)
Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion
Q Yu et al. Genes Dev. 1999.
Abstract
The cell surface hyaluronan receptor CD44 promotes tumor growth and metastasis by mechanisms that remain poorly understood. We show here that CD44 associates with a proteolytic form of the matrix metalloproteinase-9 (MMP-9) on the surface of mouse mammary carcinoma and human melanoma cells. CD44-associated cell surface MMP-9 promotes cell-mediated collagen IV degradation in vitro and mediates tumor cell invasion of G8 myoblast monolayers. Several distinct CD44 isoforms coprecipitate with MMP-9 and CD44/MMP-9 coclustering is observed to be dependent on the ability of CD44 to form hyaluronan-induced aggregates. Disruption of CD44/MMP-9 cluster formation, by overexpression of soluble or truncated cell surface CD44, is shown to inhibit tumor invasiveness in vivo. Our observations indicate that CD44 serves to anchor MMP-9 on the cell surface and define a mechanism for CD44-mediated tumor invasion.
Figures
Figure 1
Comparison of growth and invasiveness of tumors derived from TA3 CD44 transfectants. (A) Tumor weight 21 days following subcutaneous injection of 2 × 106 viable cells into syngeneic A/jax mice. At least six animals were injected with each transfectant. Mean weight ±
s.d.
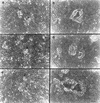
of tumors derived from the different TA3 transfectants are shown. (B) Histology of a representative TA3c (a), TA3sCD44v6-10 (b), and TA3sCD44v6-10R43A (c) tumor. The TA3c and TA3sCD44v6-10R43A tumors (a,c) show irregular borders and invasion of adjacent adipose and muscle tissue. In contrast, the TA3sCD44v6-10 tumor (b) is characterized by smooth borders and absence of invasion. Bars, 400 μm.
Figure 1
Comparison of growth and invasiveness of tumors derived from TA3 CD44 transfectants. (A) Tumor weight 21 days following subcutaneous injection of 2 × 106 viable cells into syngeneic A/jax mice. At least six animals were injected with each transfectant. Mean weight ±
s.d.
of tumors derived from the different TA3 transfectants are shown. (B) Histology of a representative TA3c (a), TA3sCD44v6-10 (b), and TA3sCD44v6-10R43A (c) tumor. The TA3c and TA3sCD44v6-10R43A tumors (a,c) show irregular borders and invasion of adjacent adipose and muscle tissue. In contrast, the TA3sCD44v6-10 tumor (b) is characterized by smooth borders and absence of invasion. Bars, 400 μm.
Figure 2
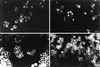
Binding and internalization of Fl–HA by TA3c and TA3CD44H cells. Twenty-four hours (a,c) and 48 hr (b,d) after incubation with Fl–HA (20 μg/ml), capping and endocytotic vesicles were observed in TA3c cells, respectively (a,b, arrows); although TA3CD44H cells bind FL–HA, they fail to display capping or endocytotic vesicles at corresponding time points (c,d, respectively). Bar, 40 μm.
Figure 3
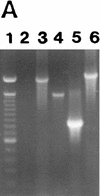
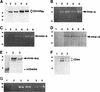
Expression of MMPs at the RNA and protein levels. (A) RT–PCR analysis of MMP transcripts in mRNA derived from TA3c cells. (Lane 1) Ladder of 100-bp DNA, ranging from 200–1500 bp; the top band corresponds to 2 kb; (lane 2) negative control PCR; (lane 3) MMP-2; (lane 4) MMP-3; (lane 5) MMP-7; (lane 6) MMP-9. (B) Zymograms of TA3c and TA3sCD44v6-10, TA3CD44tr, and TA3CD44H transfectant-derived supernatants, lysates, and crude membrane preparations. Gelatin (a,c,d) and β-casein (b) gels were used to test, respectively gelatinase A/MMP-2 (open arrow) and B/MMP-9 (solid arrow) and stromelysin (MMP-3, small arrows) activity in serum-free supernatants (a,b), water-soluble whole cell lysates (c) and 1% Triton-soluble crude membrane extracts of TA3 transfectants (d). Two independent isolates of each transfectant were tested. TA3 cells were transfected with the following: (Lanes 1,2) CD44tr; (lanes 3,4) CD44H; (lanes 5,6) sCD44v6-10; (lanes 7,8) sICAM-1; (lanes 9,10) sCD44v6-10R43A; (lanes 11,12), vector only. Molecular markers corresponding to 83 and 49 kD are indicated by arrowheads at left in a, c, and d; a molecular marker corresponding to 49 kD is indicated by an arrowhead at left in b. Bands corresponding to specific MMPs are shown.
Figure 3
Expression of MMPs at the RNA and protein levels. (A) RT–PCR analysis of MMP transcripts in mRNA derived from TA3c cells. (Lane 1) Ladder of 100-bp DNA, ranging from 200–1500 bp; the top band corresponds to 2 kb; (lane 2) negative control PCR; (lane 3) MMP-2; (lane 4) MMP-3; (lane 5) MMP-7; (lane 6) MMP-9. (B) Zymograms of TA3c and TA3sCD44v6-10, TA3CD44tr, and TA3CD44H transfectant-derived supernatants, lysates, and crude membrane preparations. Gelatin (a,c,d) and β-casein (b) gels were used to test, respectively gelatinase A/MMP-2 (open arrow) and B/MMP-9 (solid arrow) and stromelysin (MMP-3, small arrows) activity in serum-free supernatants (a,b), water-soluble whole cell lysates (c) and 1% Triton-soluble crude membrane extracts of TA3 transfectants (d). Two independent isolates of each transfectant were tested. TA3 cells were transfected with the following: (Lanes 1,2) CD44tr; (lanes 3,4) CD44H; (lanes 5,6) sCD44v6-10; (lanes 7,8) sICAM-1; (lanes 9,10) sCD44v6-10R43A; (lanes 11,12), vector only. Molecular markers corresponding to 83 and 49 kD are indicated by arrowheads at left in a, c, and d; a molecular marker corresponding to 49 kD is indicated by an arrowhead at left in b. Bands corresponding to specific MMPs are shown.
Figure 4
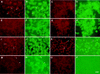
Colocalization of CD44 and MMP-9 in TA3 cells. TA3 transfectants were incubated with rat anti-mouse CD44 mAb IM7.8 and polyclonal goat anti-mouse MMP-9 antibody followed by FITC-labeled anti-rat antibody (green fluorescence) and TRITC-labeled donkey antigoat antibody (red fluorescence). (A,B) TA3c cells; (C,D) hyaluronidase-treated TA3c cells; (E,F) TA3sCD44v6-10 transfectants; (G,H) TA3CD44tr transfectants; (I,J) TA3sCD44v6-10R43A transfectants; (K,L) TA3c cells stained with anti-CD44 mAb KM201, which fails to recognize CD44/MMP-9 containing clusters; (M,N) TA3c cells stained with anti-TIMP-1 mAb (M), which fails to recognize CD44/MMP-9 clusters (N). (O,P) TA3c cells incubated with secondary antibodies only. (Arrows) Corresponding and noncorresponding capping. Bar, 43 μm.
Figure 5
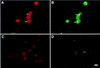
Anti-CD44 antibody-induced CD44-MMP-9 cocapping. TA3 cells were detached with EDTA, replated, and incubated with KM201 mAb, and FITC-labeled rabbit-anti-rat secondary antibody. The cells were then fixed and incubated with anti-MMP-9 antibody and TRITC-labeled donkey anti-goat antibodies. Staining shown is with anti-MMP-9 antibody (A); anti-CD44 mAb (B); secondary TRITC labeled antibody (C); secondary FITC-labeled antibody (D). Bar, 22 μm.
Figure 6
Interaction between CD44 and MMP-9. (A) Western blot analysis of CD44Rgs derived from supernatants of TA3 cells transfected with corresponding CD44–Igs. Receptor globulins were immunoprecipitated with protein A beads and blotted with anti-CD44 mAb IM7.8. Supernatants were from TA3c cells (lane 1), and from TA3 cells transfected with immunoglobulin fusions of CD44H (lane 2), CD44v7 (lane 3), CD44v8-10 (lane 4), and CD44 v7-10 (lane 5). (B) Gelatin zymogram of TA3 cell lysates immunoprecipitated with protein A-purified human IgG (lane 1), CD44HRg (lane 2), CD44v7Rg (lane 3), CD44v8-10Rg (lane 4), CD44v7-10Rg (lane 5). (C,D) Gelatin zymogram (C), and Western blot analysis (D) of immunoprecipitates from TA3 cell lysates by use of anti-ICAM-1 mAb HB233 (lanes 1,2), anti-CD44 mAb KM81 (lanes 3,4), and anti-CD44 mAb KM201 (lanes 5,6). Immunoprecipitates in D were blotted with anti-MMP-9 antibody. (E) Supernatants of TA3 cells transiently transfected with constructs encoding soluble CD40-v5 (lane 1) and MMP-9-v5 (lanes 2,3) fusion proteins blotted with anti-v5 mAb. (F) Western blot analysis with anti-CD44 mAb IM7.8 of anti-v5 mAb immunoprecipitates from lysates of TA3 cells transiently transfected with v5-tagged soluble CD40 (lane 1) and MMP-9 (lanes 2,3, corresponding to two independent transfectants) constructs. (G) Gelatin zymogram of TA3 cell lysates immunoprecipitated with CD40Rg (lanes 1,2), CD44HRg (lanes 3,4), CD44HR43ARg (lanes 5,6). In each case, Rg fusion proteins from two independent transfectants were used. (Arrowheads) Molecular mass markers of 203, 116, and 83 kD (A), 116, 83, and 49 kD (B_–_F) and 116 and 83 kD (G). Bands corresponding to CD44Rgs, MMP-9, sCD40v5, and CD44 are indicated.
Figure 7
CD44-dependent cell-mediated degradation of collagen IV. Twenty-four-well plates were coated with 3H-labeled collagen type IV (5000 cpm/well), and TA3 and MC transfectants were seeded onto the plates at 2 × 105 cell/well. Following a 16 hr incubation at 37°C, cell culture supernatants were recovered, centrifuged, and the radiolabel quantified from one-fifth of each supernatant in a β-counter. Cells were either untreated or preincubated with antibody, MMP-inhibitor peptides, or phenantroline as described in Materials and Methods. (A) Collagen IV degradation by MCwt and MCCD44 transfectants pretreated or not with MMP-3 inhibitor peptide, MMP-inhibitor peptide I, anti-MMP-2, and anti-MMP-9 mAb; (B) TA3c and TA3sCD44v6-10 cells pretreated or not with 1-10 phenanthroline, aprotinin, anti-ICAM mAb HB233, blocking anti-CD44 mAb KM201, and weakly blocking anti-CD44 mAb IM7.8. (C) Collagen degradation by two independent TA3c and TA3sCD44v6-10 isolates transfected with scrambled (vector) or antisense MMP-9 cDNA. The results are expressed as the mean ±
s.d.
of triplicate values.
Figure 7
CD44-dependent cell-mediated degradation of collagen IV. Twenty-four-well plates were coated with 3H-labeled collagen type IV (5000 cpm/well), and TA3 and MC transfectants were seeded onto the plates at 2 × 105 cell/well. Following a 16 hr incubation at 37°C, cell culture supernatants were recovered, centrifuged, and the radiolabel quantified from one-fifth of each supernatant in a β-counter. Cells were either untreated or preincubated with antibody, MMP-inhibitor peptides, or phenantroline as described in Materials and Methods. (A) Collagen IV degradation by MCwt and MCCD44 transfectants pretreated or not with MMP-3 inhibitor peptide, MMP-inhibitor peptide I, anti-MMP-2, and anti-MMP-9 mAb; (B) TA3c and TA3sCD44v6-10 cells pretreated or not with 1-10 phenanthroline, aprotinin, anti-ICAM mAb HB233, blocking anti-CD44 mAb KM201, and weakly blocking anti-CD44 mAb IM7.8. (C) Collagen degradation by two independent TA3c and TA3sCD44v6-10 isolates transfected with scrambled (vector) or antisense MMP-9 cDNA. The results are expressed as the mean ±
s.d.
of triplicate values.
Figure 7
CD44-dependent cell-mediated degradation of collagen IV. Twenty-four-well plates were coated with 3H-labeled collagen type IV (5000 cpm/well), and TA3 and MC transfectants were seeded onto the plates at 2 × 105 cell/well. Following a 16 hr incubation at 37°C, cell culture supernatants were recovered, centrifuged, and the radiolabel quantified from one-fifth of each supernatant in a β-counter. Cells were either untreated or preincubated with antibody, MMP-inhibitor peptides, or phenantroline as described in Materials and Methods. (A) Collagen IV degradation by MCwt and MCCD44 transfectants pretreated or not with MMP-3 inhibitor peptide, MMP-inhibitor peptide I, anti-MMP-2, and anti-MMP-9 mAb; (B) TA3c and TA3sCD44v6-10 cells pretreated or not with 1-10 phenanthroline, aprotinin, anti-ICAM mAb HB233, blocking anti-CD44 mAb KM201, and weakly blocking anti-CD44 mAb IM7.8. (C) Collagen degradation by two independent TA3c and TA3sCD44v6-10 isolates transfected with scrambled (vector) or antisense MMP-9 cDNA. The results are expressed as the mean ±
s.d.
of triplicate values.
Figure 8
G8 myoblast monolayer invasion by MC cell transfectants. G8 myoblast monolayers were prepared and fixed as described in Materials and Methods. MC cell transfectants were seeded onto the monolayers in six-well plates at 5 × 103 cells/well with or without preincubation with antibody, phenanthroline, and MMP-inhibitor peptides. After 7–10 days of incubation in the presence or absence of these reagents, cell invasiveness was documented under an inverted microscope. G8 monolayer invasion was as follows: MC cells transfected with vector only (a); (b) MC44H cells; (c_–_f) MC44H cells pretreated with MMP inhibitor peptide I (c), MMP-3 inhibitor peptide (d), anti-MMP-9 mAb (e), and isotype matched mouse IgG (f). Bar, 128 μm.
Similar articles
- Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function.
Yu Q, Toole BP, Stamenkovic I. Yu Q, et al. J Exp Med. 1997 Dec 15;186(12):1985-96. doi: 10.1084/jem.186.12.1985. J Exp Med. 1997. PMID: 9396767 Free PMC article. - CD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activity.
Montgomery N, Hill A, McFarlane S, Neisen J, O'Grady A, Conlon S, Jirstrom K, Kay EW, Waugh DJ. Montgomery N, et al. Breast Cancer Res. 2012 May 23;14(3):R84. doi: 10.1186/bcr3199. Breast Cancer Res. 2012. PMID: 22621373 Free PMC article. - The role of CD44 as a cell surface hyaluronan receptor during tumor invasion of connective tissue.
Knudson W. Knudson W. Front Biosci. 1998 Jul 1;3:d604-15. doi: 10.2741/a305. Front Biosci. 1998. PMID: 9634543 Review. - Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression.
Bourguignon LY, Shiina M, Li JJ. Bourguignon LY, et al. Adv Cancer Res. 2014;123:255-75. doi: 10.1016/B978-0-12-800092-2.00010-1. Adv Cancer Res. 2014. PMID: 25081533 Free PMC article. Review.
Cited by
- Neutrophil Adhesion and the Release of the Free Amino Acid Hydroxylysine.
Galkina SI, Fedorova NV, Ksenofontov AL, Serebryakova MV, Golenkina EA, Stadnichuk VI, Baratova LA, Sud'ina GF. Galkina SI, et al. Cells. 2021 Mar 5;10(3):563. doi: 10.3390/cells10030563. Cells. 2021. PMID: 33807594 Free PMC article. - Ectopic NGAL expression can alter sensitivity of breast cancer cells to EGFR, Bcl-2, CaM-K inhibitors and the plant natural product berberine.
Chappell WH, Abrams SL, Franklin RA, LaHair MM, Montalto G, Cervello M, Martelli AM, Nicoletti F, Candido S, Libra M, Polesel J, Talamini R, Milella M, Tafuri A, Steelman LS, McCubrey JA. Chappell WH, et al. Cell Cycle. 2012 Dec 1;11(23):4447-61. doi: 10.4161/cc.22786. Epub 2012 Nov 16. Cell Cycle. 2012. PMID: 23159854 Free PMC article. - Overexpression of matrix metalloproteinase 9 in tumor epithelial cells correlates with colorectal cancer metastasis.
Zuzga DS, Gibbons AV, Li P, Lubbe WJ, Chervoneva I, Pitari GM. Zuzga DS, et al. Clin Transl Sci. 2008 Sep;1(2):136-41. doi: 10.1111/j.1752-8062.2008.00037.x. Clin Transl Sci. 2008. PMID: 20443834 Free PMC article. - 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and lung cancer.
Tai HH, Tong M, Ding Y. Tai HH, et al. Prostaglandins Other Lipid Mediat. 2007 May;83(3):203-8. doi: 10.1016/j.prostaglandins.2007.01.007. Epub 2007 Jan 17. Prostaglandins Other Lipid Mediat. 2007. PMID: 17481556 Free PMC article. Review.
References
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. - PubMed
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. - PubMed
- Basbaum C, Werb Z. Focalized proteolysis: Spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous