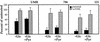Protective function of von Hippel-Lindau protein against impaired protein processing in renal carcinoma cells - PubMed (original) (raw)
Protective function of von Hippel-Lindau protein against impaired protein processing in renal carcinoma cells
M Gorospe et al. Mol Cell Biol. 1999 Feb.
Abstract
The absence of functional von Hippel-Lindau (VHL) tumor suppressor gene leads to the development of neoplasias characteristic of VHL disease, including renal cell carcinoma (RCC). Here, we compared the sensitivity of RCC cells lacking VHL gene function with that of RCC cells expressing the wild-type VHL gene (wtVHL) after exposure to various stresses. While the response to most treatments was not affected by the VHL gene status, glucose deprivation was found to be much more cytotoxic for RCC cells lacking VHL gene function than for wtVHL-expressing cells. The heightened sensitivity of VHL-deficient cells was not attributed to dissimilar energy requirements or to differences in glucose uptake, but more likely reflects a lesser ability of VHL-deficient cells to handle abnormally processed proteins arising from impaired glycosylation. In support of this hypothesis, other treatments which act through different mechanisms to interfere with protein processing (i.e., tunicamycin, brefeldin A, and azetidine) were also found to be much more toxic for VHL-deficient cells. Furthermore, ubiquitination of cellular proteins was elevated in VHL-deficient cells, particularly after glucose deprivation, supporting a role for the VHL gene in ubiquitin-mediated proteolysis. Accordingly, the rate of elimination of abnormal proteins was lower in cells lacking a functional VHL gene than in wtVHL-expressing cells. Thus, pVHL appears to participate in the elimination of misprocessed proteins, such as those arising in the cell due to the unavailability of glucose or to other stresses.
Figures
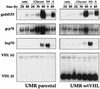
FIG. 1
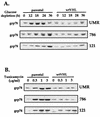
Northern blot analysis of expression of stress-responsive genes. UMR cells either lacking VHL function (UMR parental) or stably expressing wild-type VHL (UMR wtVHL) were treated as indicated. Total RNA was processed as described in Materials and Methods, and representative Northern blots indicate the levels of gadd153, grp78, hsp70, and VHL mRNA. VHL (e), endogenous VHL mRNA, about 5-kb long; VHL (t), VHL transcript from the pCEP4VHL vector overexpressed in these cells, about 1-kb long; untr., untreated; −Glucose, cells cultured in glucose-free medium; −S, cells cultured in serum-free medium; HS, cells subjected to heat shock.
FIG. 2
Effect of hypoxia and glucose deprivation on alterations in cell number. UMR cells were plated in 60-mm dishes and grown to a density of 100,000 cells per plate in complete medium. (A) Cells were placed in a hypoxia chamber, and cell numbers were determined at the times indicated. (B) Cell medium was replaced with 2 ml of glucose-free DMEM supplemented with 10% FBS. This contributed 100 mg of glucose (200 μg of glucose/plate, about 2 ng/cell) per ml. The number of cells per plate at each time point was determined in duplicate with a hemacytometer. Values represent the mean ± the standard error of the mean (SEM) for three independent experiments. Symbols: ■, UMR parental cells; □, UMR wtVHL cells. Untreated control plates were seeded at the same density and cultured in 2 ml of complete medium, and cell numbers indicated normal, logarithmic growth for at least 4 days (data not shown).
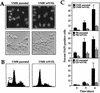
FIG. 3
Effect of glucose deprivation on three pairs of RCC lines, each with a VHL-proficient and -deficient counterpart. (A) UMR cells (either parental or expressing wtVHL) were cultured in glucose-free medium for 3 days and then stained with the DNA dye DAPI. Nuclei were visualized under fluorescence microscopy (top row). Untreated control cells also displayed homogeneous DAPI staining (data not shown). Note the presence of condensed and fragmented nuclei in the parental cell population, while most nuclei of wtVHL-expressing cells are homogeneously stained. Morphological differences were also visible under light microscopy, with parental cells exhibiting distinct membrane blebbing under phase-contrast microscopy (bottom row). (B) FACS distribution of UMR cells, each with a different VHL status. Three days after culture in glucose-free medium, UMR parental and UMR wtVHL cells were subjected to FACS analysis, and the resulting histograms are shown. A sub-G1 population, characteristic of apoptosis, is indicated with an open arrow. (C) The RCC lines UMR, 786, and 121 were subjected to glucose deprivation for the times indicated and then stained with DAPI, and the condensed and/or fragmented nuclei were scored. Solid bars, parental cells; hatched bars, wtVHL-expressing cells.
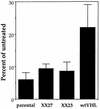
FIG. 4
Effect of glucose deprivation on UMR cells expressing C-terminally truncated pVHL. UMR cells that lacked VHL (parental), expressed wtVHL (wtVHL), or expressed a mutant VHL cDNA carrying a deletion at nucleotide position 737 that rendered a C-terminally truncated protein (clonal lines XX23 and XX27) were cultured in glucose-free medium for 4 days. At the end of the treatment period, the cultures were subjected to clonogenicity assay as described in Materials and Methods. Percentages were calculated relative to untreated, time-matched controls. Values represent the mean ± the SEM for at least three independent experiments.
FIG. 5
Effect of glucose deprivation on long-term survival of RCC cells each with a different VHL status. UMR, 786, and 121 cells (both parental and expressing wtVHL) were cultured in glucose-deprived medium for the times indicated, and then their long-term survival was assayed by using a clonogenic assay as described in Materials and Methods. Percentages were calculated relative to untreated, time-matched controls. In each experiment, treatments were done in duplicate, and values represent the mean ± the SEM for at least three independent experiments.
FIG. 6
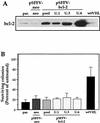
Influence of bcl-2 overexpression in glucose deprivation toxicity. (A) Western blot analysis of bcl-2 expression in transfected UMR cells. UMR cells were transfected with the bcl-2 expression vector pSFFV-bcl-2 to obtain a pool population (pool) or isolated clones (U.1, U.3, and U.6) of bcl-2-expressing cells. neo, cells transfected with the “empty” vector control pSFFV-neo. Different levels of bcl-2 were expressed in each case. par, parental. (B) The sensitivity of each clone to the toxic influence of a 3-day glucose deprivation period was measured in a colony formation assay. In each experiment, treatments were done in duplicate, and the values represent the mean ± the SEM for at least three independent experiments.
FIG. 7
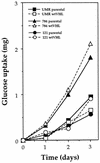
Glucose uptake in RCC cells. UMR, 786, and 121 cells were cultured in complete medium, and the rate of glucose uptake was determined by analyzing aliquots of medium collected at the times indicated. The glucose concentrations were determined as described in Materials and Methods.
FIG. 8
Effect of pyruvate on the toxicity by glucose deprivation. UMR, 786, and 121 cells, plated at a density of 30,000 cells/well in 6-well cluster plates, were cultured for 48 h in glucose-free medium alone (solid bars) or supplemented with 10 mM sodium pyruvate (hatched bars). At the end of the treatment period, the cultures were subjected to clonogenicity assay as described in Materials and Methods. Percentages were calculated relative to untreated, time-matched controls. Pyruvate alone had no influence on clonogenicity relative to untreated cells. In each experiment, treatments were done in duplicate, and the values represent the mean ± the SEM for at least three independent experiments.
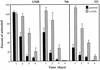
FIG. 9
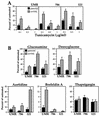
Effect of inhibitors of posttranslational protein processing on RCC cells. Parental (solid bars) and wtVHL-expressing (hatched bars) UMR, 786, and 121 cells, were plated at a density of 30,000 cells/well in 6-well cluster plates and cultured for 48 h in complete medium, either alone or supplemented with tunicamycin (A) or a variety of drug treatments affecting protein processing: glucosamine (10 mM), 2-deoxyglucose (10 mM), azetidine (3 mM), brefeldin A (0.3 μg/ml) and thapsigargin (3 μM) (B), with the exception of UMR cells, which were treated with 2-deoxyglucose (10 mM) for 72 h. At the end of each treatment period, cultures were subjected to clonogenic assay as described in Materials and Methods. Percentages were calculated relative to untreated, time-matched controls. Dimethyl sulfoxide alone had no influence on clonogenicity relative to untreated cells. Treatments were done in duplicate, and the values represent the mean ± the SEM for at least three independent experiments.
FIG. 10
Expression of grp78 in RCC cells. Representative Western blot analysis of grp78 expression in VHL-deficient (parental) and wtVHL-expressing (wtVHL) UMR, 786, and 121 cells that were cultured in glucose-depleted medium for the times indicated (A) or in complete medium with various concentrations of tunicamycin for 12 h (B). Protein loading was the same in all lanes (data not shown).
FIG. 11
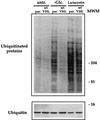
Western blot analysis of ubiquitinated proteins and ubiquitin in RCC cells. (Upper panel) Parental (par.) and wtVHL-expressing 786 cells were either left untreated, subjected to glucose-deprivation for 48 h, or treated with lactacystin (10 μM) for 16 h. Whole-cell lysates were then electrophoresed in SDS–7% polyacrylamide gels and subjected to Western blot analysis to detect the presence of ubiquitin by using a monoclonal anti-ubiquitin antibody. (Lower panel) Electrophoresis in SDS–15% polyacrylamide gels was used to visualize free ubiquitin (8.5 kDa). Protein loading was the same in all lanes (not shown). MWM, molecular weight marker, indicating the size in kilodaltons.
FIG. 12
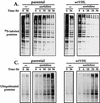
Elimination of 35S-labeled proteins synthesized in the presence of amino acid analogs. (A) 786 cells either were left untreated or were pretreated with 10 mM azetidine for 1 h and incubated with [35S]methionine for an additional 5 h (as described in Materials and Methods). Then the labeling medium was removed and replaced with regular culture medium without drugs. At different times thereafter (0, 6, 18, 24, and 36 h), protein extracts from each treatment group were prepared and electrophoresed through SDS–12% polyacrylamide gels. Gels were dried, and radiolabeled proteins were visualized with a PhosphorImager. parental, VHL-deficient cells; wtVHL, cells expressing wtVHL. (B) 786 cells either were left untreated or were pretreated with 10 mM azetidine for 1 h and then incubated with [35S]methionine for an additional 5 h. At the times indicated after removal of the medium containing [35S]methionine (with or without azetidine), protein lysates were prepared, and TCA-precipitable counts were determined as described in Materials and Methods. (C) 786 cells either were left untreated or were treated with 10 mM azetidine for 6 h and then placed in medium without drugs. At different times thereafter (0, 6, 18, 24, and 36 h), protein extracts were prepared and subjected to Western blot analysis for the detection of ubiquitinated proteins as described in Materials and Methods.
FIG. 12
Elimination of 35S-labeled proteins synthesized in the presence of amino acid analogs. (A) 786 cells either were left untreated or were pretreated with 10 mM azetidine for 1 h and incubated with [35S]methionine for an additional 5 h (as described in Materials and Methods). Then the labeling medium was removed and replaced with regular culture medium without drugs. At different times thereafter (0, 6, 18, 24, and 36 h), protein extracts from each treatment group were prepared and electrophoresed through SDS–12% polyacrylamide gels. Gels were dried, and radiolabeled proteins were visualized with a PhosphorImager. parental, VHL-deficient cells; wtVHL, cells expressing wtVHL. (B) 786 cells either were left untreated or were pretreated with 10 mM azetidine for 1 h and then incubated with [35S]methionine for an additional 5 h. At the times indicated after removal of the medium containing [35S]methionine (with or without azetidine), protein lysates were prepared, and TCA-precipitable counts were determined as described in Materials and Methods. (C) 786 cells either were left untreated or were treated with 10 mM azetidine for 6 h and then placed in medium without drugs. At different times thereafter (0, 6, 18, 24, and 36 h), protein extracts were prepared and subjected to Western blot analysis for the detection of ubiquitinated proteins as described in Materials and Methods.
Similar articles
- Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein.
Lee S, Neumann M, Stearman R, Stauber R, Pause A, Pavlakis GN, Klausner RD. Lee S, et al. Mol Cell Biol. 1999 Feb;19(2):1486-97. doi: 10.1128/MCB.19.2.1486. Mol Cell Biol. 1999. PMID: 9891082 Free PMC article. - Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease.
Zatyka M, da Silva NF, Clifford SC, Morris MR, Wiesener MS, Eckardt KU, Houlston RS, Richards FM, Latif F, Maher ER. Zatyka M, et al. Cancer Res. 2002 Jul 1;62(13):3803-11. Cancer Res. 2002. PMID: 12097293 - The von Hippel-Lindau tumor suppressor gene and kidney cancer.
Kaelin WG Jr. Kaelin WG Jr. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6290S-5S. doi: 10.1158/1078-0432.CCR-sup-040025. Clin Cancer Res. 2004. PMID: 15448019 Review. - Von Hippel-Lindau disease and sporadic renal cell carcinoma.
Zbar B. Zbar B. Cancer Surv. 1995;25:219-32. Cancer Surv. 1995. PMID: 8718521 Review.
Cited by
- Differences in regulation of tight junctions and cell morphology between VHL mutations from disease subtypes.
Bangiyeva V, Rosenbloom A, Alexander AE, Isanova B, Popko T, Schoenfeld AR. Bangiyeva V, et al. BMC Cancer. 2009 Jul 14;9:229. doi: 10.1186/1471-2407-9-229. BMC Cancer. 2009. PMID: 19602254 Free PMC article. - The von Hippel-Lindau protein sensitizes renal carcinoma cells to apoptotic stimuli through stabilization of BIM(EL).
Guo Y, Schoell MC, Freeman RS. Guo Y, et al. Oncogene. 2009 Apr 23;28(16):1864-74. doi: 10.1038/onc.2009.35. Epub 2009 Mar 23. Oncogene. 2009. PMID: 19305426 Free PMC article. - Glucocorticoids suppress renal cell carcinoma progression by enhancing Na,K-ATPase beta-1 subunit expression.
Huynh TP, Barwe SP, Lee SJ, McSpadden R, Franco OE, Hayward SW, Damoiseaux R, Grubbs SS, Petrelli NJ, Rajasekaran AK. Huynh TP, et al. PLoS One. 2015 Apr 2;10(4):e0122442. doi: 10.1371/journal.pone.0122442. eCollection 2015. PLoS One. 2015. PMID: 25836370 Free PMC article. - Oxygen-dependent ubiquitination and degradation of hypoxia-inducible factor requires nuclear-cytoplasmic trafficking of the von Hippel-Lindau tumor suppressor protein.
Groulx I, Lee S. Groulx I, et al. Mol Cell Biol. 2002 Aug;22(15):5319-36. doi: 10.1128/MCB.22.15.5319-5336.2002. Mol Cell Biol. 2002. PMID: 12101228 Free PMC article. - A bone-seeking clone exhibits different biological properties from the ACHN parental human renal cell carcinoma in vivo and in vitro.
Wang J, Chen A, Yang C, Zeng H, Qi J, Guo FJ. Wang J, et al. Oncol Rep. 2012 Apr;27(4):1104-10. doi: 10.3892/or.2011.1572. Epub 2011 Nov 30. Oncol Rep. 2012. PMID: 22139406 Free PMC article.
References
- Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical